ARTÍCULO
Palynological characteristics of the heterostylous subspecies of Linum mucronatum Bertol.
S. M. TALEBI1, F. FARAHANI2, M. SHEIDAI2 & Z. NOORMOHAMMADI3
1 Department of Biology, Faculty of Sciences, Arak University, Arak IR-38156-8-8349, Iran
2 Faculty of Biological Sciences, Shahid Beheshti University, Tehran, Iran
3 Department of Biology, School of Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran
Author for correspondence: S. M. Talebi (Seyedmehdi_Talebi@Yahoo.com)
Editor: T. Garnatje
ABSTRACT
Palynological characteristics of the heterostylous subspecies of Linum mucronatum Bertol.— Linum mucronatum is a heterostylous species from sect. Syllinum with four subspecies in Iran. The present study examines palynological characteristics of the heterostylous subspecies of Linum mucronatum, pollen characters of brevistylous individuals (pins) as well as longistylous individuals (thrums) of these plants by scanning electron microscope and light microscope using the prolonged acetolysis procedure. Sixteen qualitative and quantitative characters were investigated. Pollen equatorial shapes varied between pin and thrum individuals of each subspecies with the exception of L. mucronatum subsp. assyriacum. Pollen sculptures varied between pin and thrum samples of each subspecies and were seen in the gemmate, clavate and baculate shapes. In addition, quantitative palynological characters differed between plants and ANOVA test showed significant variations for traits such as equatorial length, colpi width and apocolpium diameter. Hetrostylous individuals of each subspecies were separated from others in the UPGMA tree and also in the PCO and PCA plots. This study confirmed variations in pollen features between pin and thrum individuals of each subspecies.
KEYWORDS: heterostyly; Linum mucronatum; pollen characters; thrum/pin individuals.
Características palinológicas de las subspecies de Linum mucronatum Bertol. con heterostilia
RESUMEN
Características palinológicas de las subspecies de Linum mucronatum Bertol. con heterostilia.— Linum mucronatum es una especie con heterostilia, que pertenece a la sección Syllinum del género Linum, y tiene cuatro subespecies en Irán. En el presente estudio se examinan las características palinológicas de las subespecies heterostilas de Linum mucronatum Bertol., así como los caracteres polínicos de individuos de los morfos brevistilo (pin) y longistilo (thrum) de estas plantas, mediante microscopía electrónica de scanning y microscopía óptica usando el método de acetolisis prolongada. Se estudiaron un total de 16 caracteres cualitativos y cuantitativos. La forma ecuatorial del polen varía entre los morfos pin y thrum en todas las subspecies, excepto en L. mucronatum subsp. assyriacum. La ornamentación también varía entre las muestras de morfos pin y thrum de cada subespecie, en los que se puede observar polen gemado, clavado y baculado. En algunos caracteres palinológicos cuantitativos, se encontraron también diferencias entre morfos y el test de ANOVA muestra que son significativas en cuanto a la longitud ecuatorial, la anchura de los colpos y el diámetro del apocolpio. Los individuos heterostilos de cada susbespecie aparecen separados en el árbol UPGMA y también en los gráficos de PCO y PCA. Este estudio confirma las diferencias en las características del polen entre individuos pin y thrum de cada una de las subespecies.
PALABRAS CLAVE: características polínicas; heterostilia; individuos thrum/pin; Linum mucronatum.
Recibido: 27/10/2013 / Aceptado: 12/05/2014
Cómo citar este artículo / Citation: Talebi, S. M., Farahani, F., Shedai, M. & Noormohammadi, Z. 2014. Palynological characteristics of the heterostylous subspecies of Linum mucronatum Bertol. Collectanea Botanica 33: e004. doi: http://dx.doi.org/10.3989/collectbot.2013.v33.004
Copyright: © 2014 CSIC. Este es un artículo de acceso abierto distribuido bajo los términos de la licencia Creative Commons Attribution-Non Commercial (by-nc) Spain 3.0. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial (by-nc) Spain 3.0 License.
CONTENIDOS
INTRODUCTIONTop
Among diverse sexual systems in flowering plants, heterostyly has been one of the most attractive mating systems for researchers, although heterostylous plants are the minority within angiosperms (Naiki, 2012Naiki, A. 2012. Heterostyly and the possibility of its breakdown by polyploidization. Plant Species Biology 27: 3–29.). In distylous taxa, two flower morphs are known as pin (long style/low anther) and thrum (short style/high anther). Ganders (1979Ganders, F. R. 1979. The biology of heterostyly. New Zealand Journal of Botany 17: 607–635.) reviewed the taxonomic distribution of heterostyly and listed 164 genera in 24 families. Since then, new heterostylous taxa have been reported and based on the APG III classification system (APG III, 2009APG III. 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161: 105–121.), in a way that 199 genera in 28 families in 15 orders are recognized as taxa that contain heterostylous species (Naiki, 2012Naiki, A. 2012. Heterostyly and the possibility of its breakdown by polyploidization. Plant Species Biology 27: 3–29.).
Darwin provided the first functional interpretation of the adaptive significance of heterostyly and speculated on the evolutionary pathway leading to the evolution of distyly (Darwin, 1877Darwin, C. 1877. The different forms of flowers on plants of the same species. John Murray, London.; Barrett, 2010Barrett, S. C. H. 2010. Darwin’s legacy: the forms, function and sexual diversity of flowers. Philosophical Transactions of the Royal Society of London, B, Biological Sciences 365: 351–368.). A number of elaborate studies examining morphological, genetic, physiological, ecological, pollination, inheritance, phylogenetic reconstruction and evolutionary fields have been accumulated after Darwin’s seminal work on heterostyly (Darwin, 1877Darwin, C. 1877. The different forms of flowers on plants of the same species. John Murray, London.; Dulberger, 1992Dulberger, R. 1992. Floral polymorphisms and their functional significance in the heterostylous syndrome. In: Barrett, S. C. H. (Ed.), Evolution and function of heterostyly. Springer, Berlin, Heidelberg & New York: 41–84.; Pérez-Barrales et al., 2006Pérez-Barrales, R., Vargas, P. & Arroyo, J. 2006. New evidence for the Darwinian hypothesis of heterostyly: breeding systems and pollinators in Narcissus sect. Apodanthi. New Phytologist 171: 553–567.; Barrett & Shore, 2008Barrett, S. C. H. & Shore, J. S. 2008. New insights on heterostyly: Comparative biology, ecology and genetics. In: Franklin-Tong, V. (Ed.), Self-incompatibility in flowering plants: Evolution, diversity and mechanisms. Springer-Verlag, Berlin: 3–32; Weller, 2009Weller, S. G. 2009. The different forms of flowers—what have we learned since Darwin? Botanical Journal of the Linnean Society 160: 249–261.; Cohen, 2010Cohen, J. I. 2010. ‘A case to which no parallel exists’: the influence of Darwin’s different forms of flowers. American Journal of Botany 97: 701–716.). Although morphological differences are found between species, several characteristics are shared among them. These morphological differences promote self-incompatibility to initiate outcrossing, which is essential for seed production since most heterostylous species are self-incompatible (Barrett, 1992Barrett, S. C. H. 1992. Evolution and function of heterostyly. Springer, Berlin.; Richards, 1997Richards, A. J. 1997. Plant breeding systems (2nd ed.). Chapman and Hall, London.).
Linum is the main genus of flax family (Linaceae) and it is widely distributed in the world with over 180 species (Heywood, 1993Heywood, V. H. 1993. Flowering plants of the world. Oxford University Press, Oxford.; McDill et al., 2009McDill, J., Repplinger, M., Simpson, B. B. & Kadereit, J. W. 2009.The phylogeny of Linum and Linaceae subfamily Linoideae, with implications for their systematics, biogeography and evolution of heterostyly. Systematic Botany 34: 386-405.). Several studies have described the breeding system of Linum species. These studies showed that heterostyly (distyly) is widespread in this genus (Ockendon, 1968Ockendon, D. G. 1968. Biosystematic studies. In: Linum perenne group. New Phytologist 67: 787–813.; Dulberger, 1973Dulberger, R. 1973. Distyly in Linum pubescens and L. mucronatum. Botanical Journal of the Linnean Society 66: 117–126.; Rogers, 1979Rogers, C. M. 1979. Distyly and pollen dimorphism in Linum suffruticosum (Linaceae). Plant Systematics and Evolution 131: 127–132.; Güvensen et al., 2013Güvensen, A., Seçmen, Ö. & Şenol, S. G. 2013. Heterostyly in Linum aretioides. Turkish Journal of Botany 37: 122–129.). One of the first researchers of this genus was Darwin; he revealed the existence of distyly in several species such as L. pubescens Banks & Sol., L. grandiflorum Desf., L. mucronatum Bertol., L. flavum L., L. perenne L., L. austriacum L., and L. maritimum L. (Güvensen et al., 2013Güvensen, A., Seçmen, Ö. & Şenol, S. G. 2013. Heterostyly in Linum aretioides. Turkish Journal of Botany 37: 122–129.). This feature exists in four of the five sections recognized by Ockendon & Walters (1968Ockendon, D. J. & Walters, S. M. 1968. Linum L. In: Tutin, T. G., & Heywood, V. H. (Eds.), Flora Europaea 2. Cambridge University Press, Cambridge: 206–211.), namely, Linum, Syllinum, Dasylinum and Linastrum.
Heterostylous species display some morphological and micromorphological characteristics such as the number and size of pollen grains, stamens shape, and shape and color of stigma while its surface papillae were different in pin and thrum plants (Richards & Barrett, 1992Richards, J. H. & Barrett, S. C. H. 1992. The development of heterostyly. In: Barrett, S. C. H. (Ed.), Evolution and function of heterostyly. Springer, Berlin, Heidelberg & New York: 85–127.). Similarly, in heterostylous species of Linum such as L. perenne, L. grandiflorum and L. alpinum morphological traits like exine sculpturing structure, the stigma size as well as the wall structure of the papillae, differ between the pin and thrum plants (Dulberger, 1981Dulberger, R. 1981. Dimorphic exine sculpturing in three distylous species of Linum (Linaceae). Plant Systematics and Evolution 139: 113–119.) and also in the case of nuclear genome size (Talebi et al., 2012aTalebi, S. M., Sheidai, M., Atri, M., Sharifnia, F. & Noormohammadi, Z. 2012a. Genome size, morphological and palynological variations, and heterostyly in some species of the genus Linum L. (Linaceae) in Iran. African Journal of Biotechnology 11: 16040–16054.). In other species of the genus, e.g. L. album, L. austriacum and L. glaucum, some morphological features and pollen sculpturing shape vary between thrum and pin populations (Talebi et al., 2012aTalebi, S. M., Sheidai, M., Atri, M., Sharifnia, F. & Noormohammadi, Z. 2012a. Genome size, morphological and palynological variations, and heterostyly in some species of the genus Linum L. (Linaceae) in Iran. African Journal of Biotechnology 11: 16040–16054.). Furthermore, Armbruster et al. (2006Armbruster, W. S., Pérez-Barrales, R., Arroyo, J., Edwards, M. E. & Vargas, P. 2006. Three-dimensional reciprocity of floral morphs in wild flax (Linum suffruticosum): a new twist on heterostyly. New Phytologist 171: 581–590.) found variation in distyly of Linum suffruticosum L., with styles and stamens bending and twisting, achieving a three-dimensional arrangement.
Linum mucronatum Bertol. belongs to sect. Syllinum Griseb. It is a heterostylous species with four subspecies in Iran (Sharifnia & Assadi, 2001Sharifnia, F. & Assadi, M. 2001. Flora of Iran, No. 34: Linaceae. Research Institute of Forests (Ministry of Jahad-e-Sazandegi and Rangelands, Islamic Republic of Iran), Tehran.). Linum mucronatum was reported to be a very variable species (Özcan & Zorlu, 2009Özcan, T. & Zorlu, E. 2009. A contribution to taxonomy of Turkish Linum based on seed surface patterns. Biologia (Bratislava), Section Botany 64: 723–730.), and a palynological study confirmed these interpretations (Talebi et al., 2012bTalebi, S. M., Sheidai, M., Atri, M., Sharifnia, F. & Noormohammadi, Z. 2012b. Palynological study of the genus Linum in Iran (a taxonomic review). Phytologia Balcanica 18: 293–303. ).
Pollen morphology in the genus Linum has an important taxonomic value (Saad, 1961Saad, S. I. 1961. Pollen morphology and sporoderm stratification in Linum. Grana 3: 109–129.; Erdtman, 1964Erdtman, G. 1964. Palynology. In: Turrill, N. B. (Ed.), Vistas in Botany IV. Recent Researches in Plant Taxonomy. Permagon Press, Oxford: 23–54.). Characters such as exine sculpturing have been used as diagnostic at various taxonomic levels (Xavier & Rogers, 1963Xavier, K. S. & Rogers, C. M. 1963. Pollen morphology as a taxonomic tool in Linum. Rhodora 65: 137–145.; Ockendon, 1968Ockendon, D. G. 1968. Biosystematic studies. In: Linum perenne group. New Phytologist 67: 787–813.), as well as intraspecific exine polymorphism (Dulberger, 1981Dulberger, R. 1981. Dimorphic exine sculpturing in three distylous species of Linum (Linaceae). Plant Systematics and Evolution 139: 113–119.). In the present study in order to study the effects of heterostyly on palynological characters, pollen morphological traits of pin as well as thrum individuals of four L. mucronatum subspecies were investigated in Iran.
MATERIAL AND METHODSTop
Plant samples
Plant specimens of pin and thrum individuals of four L. mucronatum subspecies, namely L. mucronatum subsp. armenum, L. mucronatum subsp. mucronatum, L. mucronatum subsp. orientale and L. mucronatum subsp. assyriacum were collected from natural populations in different regions of Iran during spring 2010 and 2011. In each locality three to four individuals were collected randomly per each morph. Details of localities and voucher numbers are given in Table 1. Vouchers have been deposited in the herbarium of Shahid Beheshti University of Tehran, Iran (HSBU).
| Table 1. Locality and herbarium voucher number of studied subspecies of Linum mucronatum. |
|
Taxa
|
Locality Voucher
|
|
L. mucronatum Bertol. subsp. mucronatum pin |
Hamedan, Avaj, 2350 m |
HSBU2011196 |
|
L. mucronatum Bertol. subsp. mucronatum thrum |
Hamedan, Avaj, 2350 m |
HSBU2011296 |
|
L. mucronatum subsp. orientale (Boiss.) P. H. Davis pin |
Zanjan, 90 km Abhar to Zanjan, 1839 m |
HSBU2011132 |
|
L. mucronatum subsp. orientale (Boiss.) P. H. Davis thrum |
Zanjan, 90 km Abhar to Zanjan, 1839 m |
HSBU2011232 |
|
L. mucronatum subsp. armenum (Bordzil.) P. H. Davis pin |
Azerbaijan, Salmas, Ghoshchi, 1557 m |
HSBU2011140 |
|
L. mucronatum subsp. armenum (Bordzil.) P. H. Davis thrum |
Azerbaijan, Salmas, Ghoshchi, 1557 m |
HSBU2011240 |
|
L. mucronatum subsp. assyriacum P. H. Davis pin |
Khuzestan, Izeh, Atabaki Park, 350 m |
HSBU2011164 |
|
L. mucronatum subsp. assyriacum P. H. Davis thrum |
Khuzestan, Izeh, Atabaki Park, 350 m |
HSBU2011264 |
|
Palynological study
Pollen grains were obtained from mature buds of heterostyled individuals. For each subspecies, three specimens were used and from each specimen, at least three to four anthers were investigated and their pollen grains prepared for scanning electron microscope (SEM) and light microscopy (LM) using the prolonged acetolysis procedure of Erdtman (1960Erdtman, G. 1960. The acetolysis method. Svensk Botanisk Tidskrift 54: 561–564.).
For LM, the pollen grains were mounted in glycerin jelly and sealed with paraffin. The polar (P) and equatorial (E) shape and length and P/E ratios were obtained under the light microscope (×1000). Three replicates were used for character measurements.
For SEM, the pollen grains were transferred directly to double-sided tape affixed stubs; they were then vacuum-coated with gold in Biorad E5200 auto sputter coater (Bio-Rad, Hercules, CA, USA) and were examined and photographed by a CamScan MV2300 scanning electron microscope at 10kV (Electron Optic Services Inc., Ottawa, Canada). The sculpturing types and dimensions, together with their fine structure, as well as colpi dimensions, apocolpium and mesocolpium length were studied (Table 2). The terminology in this paper corresponds to that the one used by Moore et al. (1991Moore, P. D., Webb, J. A. & Collinson, M. E. 1991. Pollen analysis (2nd ed.). Blackwell Scientific Publications, Oxford.).
| Table 2. Selected palynological characters of heterostylous individuals of L. mucronatum subspecies (all values are in μm). Pin: long style; Thrum: short style; N: sample size; SD: standard deviation. |
|
Taxa |
Statistical parameters |
Equatorial
shape |
Equatorial length (E) |
Polar shape |
Polar length (P) |
P/E |
Aperture shape |
Colpi length |
Colpi width |
Apocolpium length |
Aperture length |
Aperture width |
Aperture distance |
Mesocolpium |
Colpi length / width |
|
L. mucronatum subsp. assyriacum Thrum |
Mean |
elliptic-
emanate |
34.86 |
circular |
65.00 |
1.86 |
baculate and pilate |
52.24 |
3.88 |
13.40 |
1.37 |
1.35 |
0.81 |
26.34 |
13.02 |
|
N |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
|
SD |
6.61 |
3.62 |
0.33 |
9.16 |
0.60 |
0.49 |
0.51 |
0.49 |
0.34 |
0.93 |
2.75 |
|
L. mucronatum subsp. assyriacum Pin |
Mean |
elliptic-
emanate |
37.80 |
circular |
67.10 |
1.77 |
gemmate and baculate |
50.76 |
4.15 |
11.28 |
1.35 |
1.19 |
0.66 |
23.60 |
12.30 |
|
N |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
|
SD |
2.70 |
1.24 |
0.16 |
3.43 |
0.57 |
.0.78 |
0.11 |
0.09 |
0.03 |
0.82 |
1.66 |
|
L. mucronatum subsp. armenum Pin |
Mean |
obtuse-
truncate |
43.25 |
circular |
42.40 |
0.98 |
gemmate and baculate |
31.45 |
3.69 |
13.25 |
1.41 |
1.23 |
0.64 |
28.76 |
8.33 |
|
N |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
|
SD |
2.87 |
1.19 |
0.09 |
1.61 |
0.80 |
2.18 |
0.07 |
0.06 |
0.12 |
1.90 |
1.92 |
|
L. mucronatum subsp. armenum Thrum |
Mean |
elliptic-
obtuse |
39.55 |
circular |
46.98 |
1.18 |
gemmate and clavate |
39.43 |
5.21 |
17.99 |
1.18 |
1.14 |
0.45 |
32.59 |
7.69 |
|
N |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
|
SD |
6.17 |
3.87 |
0.11 |
3.57 |
0.93 |
0.36 |
0.082 |
0.11 |
0.04 |
0.53 |
1.61 |
|
L. mucronatum subsp. mucronatum Pin |
Mean |
obtuse-
truncate |
37.50 |
circular |
52.00 |
1.38 |
gemmate and clavate |
42.97 |
2.79 |
15.39 |
1.12 |
1.10 |
.51 |
28.61 |
15.47 |
|
N |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
|
SD |
3.09 |
2.90 |
0.15 |
2.68 |
0.634 |
0.71 |
0.04 |
0.05 |
0.12 |
3.38 |
4.55 |
|
L. mucronatum subsp. mucronatum Thrum |
Mean |
elliptic-
obtuse |
43.28 |
circular |
46.98 |
1.08 |
small and large gemmate |
40.91 |
7.69 |
39.79 |
1.51 |
1.41 |
0.58 |
29.92 |
5.27 |
|
N |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
|
SD |
1.48 |
1.36 |
0.045 |
2.12 |
1.67 |
0.48 |
0.18 |
0.13 |
0.16 |
1.93 |
1.33 |
|
L. mucronatum subsp. orientale Pin |
Mean |
obtuse-
truncate |
39.89 |
circular |
59.88 |
1.50 |
small and large gemmate |
45.99 |
2.67 |
16.47 |
1.41 |
1.36 |
0.76 |
27.89 |
17.31 |
|
N |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
|
SD |
0.67 |
0.95 |
0.04 |
1.45 |
0.42 |
0.88 |
0.08 |
0.08 |
0.25 |
0.72 |
2.22 |
|
L. mucronatum subsp. orientale Thrum |
Mean |
circular |
52.01 |
circular |
46.97 |
0.90 |
gemmate |
39.30 |
13.87 |
50.62 |
1.41 |
1.29 |
0.78 |
34.65 |
2.84 |
|
N |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
|
SD |
1.02 |
0.49 |
0.01 |
1.97 |
2.08 |
2.02 |
0.14 |
0.15 |
.237 |
1.63 |
0.46 |
|
Statistical analyses
For grouping the studied heterostyled individuals, data were standardized (mean = 0, variance = 1) and used for the multivariate analyses including Unweighted Paired Group using Average (UPGMA), Principal Coordinate Ordination (PCO), and Principal Coordinate Analysis (PCA), cf. Podani (2000Podani, J. 2000. Introduction to the exploration of multivariate biological data. Backhuys Publishers, Leiden.).
One-way ANOVA and t-test were employed to assess the significance of quantitative palynological difference between morph and among subspecies. Pearson’s coefficient of correlation was used to ascertain the strength of correlations between quantitative palynological characters. NTSYS v2 (Rohlf, 1998Rohlf, F. J. 1998. NTSYS-PC. Numerical taxonomy and multivariate analysis system. version 2.02i. Applied Biostatistics, Inc., New York.) and SPSS v9 (1998) were used for statistical analyses.
RESULTSTop
In the present study pollen morphological and micromorphological characters of heterostyled individuals belonging to four subspecies of L. mucronatum were investigated. Four qualitative and eleven quantitative morphological traits (totally sixteen characters) were examined. In the formerly studied subspecies, pollen grains were trizonocolpate, with three long grooves in equatorial zone and had monomorphic or polymorphic processes on their surfaces.
Pollen polar shape was similar between pin and thrum individuals of each subspecies, while this character was alike among subspecies and was seen in the shape of circular. In contrast, pollen equatorial shape was different between pin and thrum individuals of each subspecies with the exception of L. mucronatum subsp. assyriacum, the shape of which was elliptic-emanated and similar between pin and thrum individuals. Pollen equatorial shapes in pin individuals of subspecies L. mucronatum subsp. armenum, L. mucronatum subsp. mucronatum and L. mucronatum subsp. orientale were similar. These conditions were true in the case of thrum samples of L. mucronatum subsp. armenum and L. mucronatum subsp. mucronatum and were alike in the shape of elliptic-obtuse (Fig. 1). Pollen sculptures were different between pin and thrum samples of each subspecies and were seen in the gemmate and clavate shapes (L. mucronatum subsp. armenum thrum and L. mucronatum subsp. mucronatum pin), in the small and large gemmate (L. mucronatum subsp. mucronatum thrum, L. mucronatum subsp. orientale pin and L. mucronatum subsp. orientale thrum) and in the gemmate with baculate (L. mucronatum subsp. armenum pin and L. mucronatum subsp. assyriacum pin) (Fig. 2).
|
Figure 1. Electronic micrographs of pollen in studied subspecies of Linum mucronatum. Pin: long style; Thrum: short style. (A), subsp. mucronatum pin; (B), subsp. mucronatum thrum; (C), subsp. orientale pin; (D), subsp. orientale thrum; (E), subsp. assyriacum pin; (F), subsp. armenum pin. Scale bar: 20 μm.
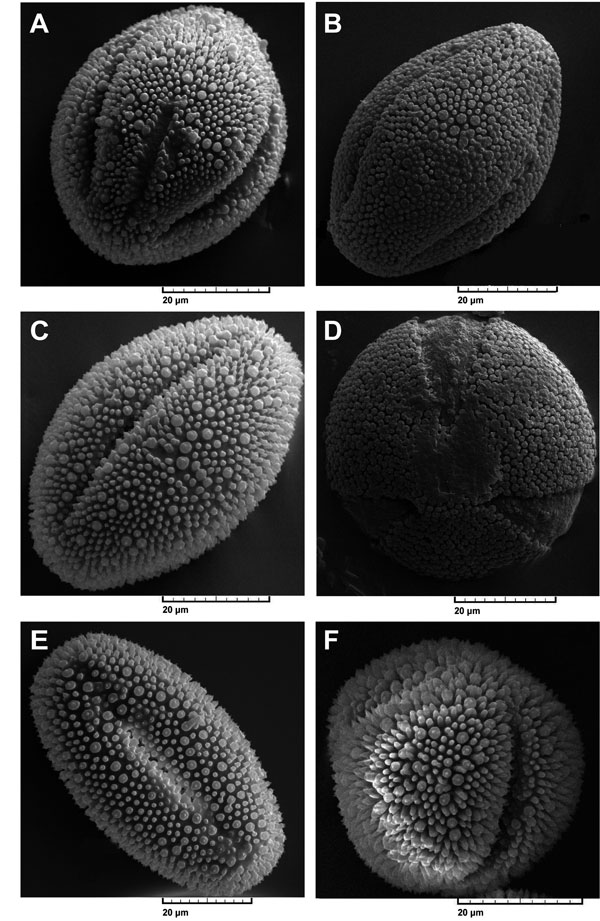
[View full size] [Descargar tamaño completo] |
|
|
Figure 2. Electronic micrographs of exine surface sculpturing in the studied subspecies of Linum mucronatum. Pin: long style, Thrum: short style. (A), subsp. armenum thrum; (B), subsp. armenum pin; (C), subsp. mucronatum thrum; (D), subsp. mucronatum pin; (E), subsp. orientale thrum; (F) subsp. orientale pin; (G), subsp. assyriacum thrum; (H), subsp. assyriacum pin. Scale bar: 20 μm.
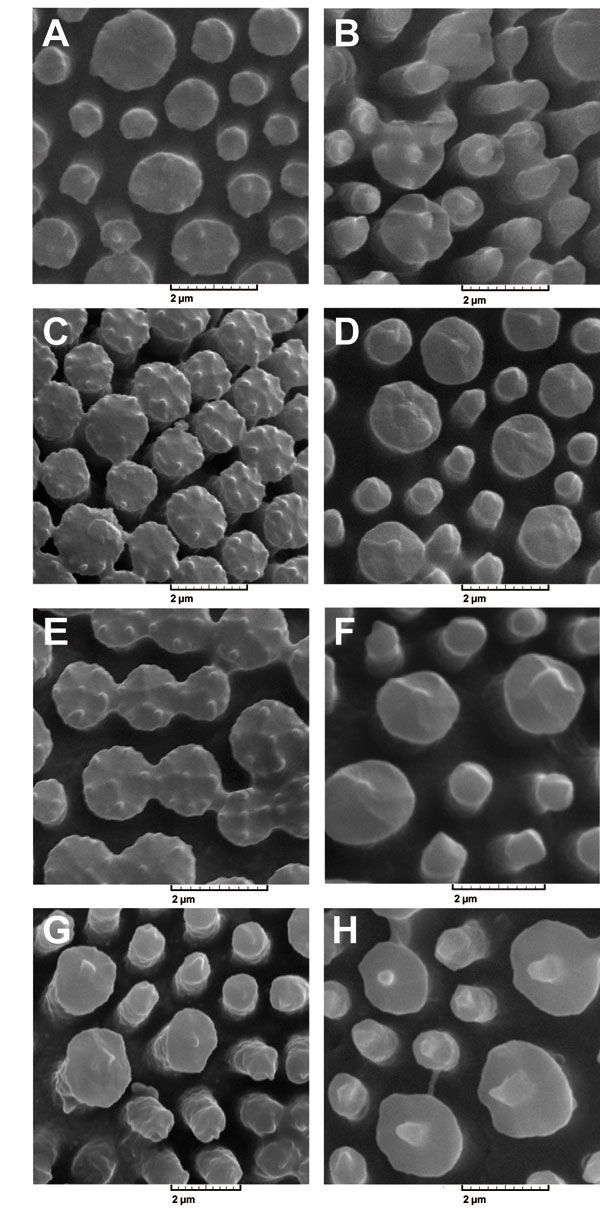
[View full size] [Descargar tamaño completo] |
|
Pollen quantitative traits were different between heterostylous individuals and subspecies. Box and whisker plots are ideal for detecting and illustrating distributions pattern of quantitative data because the central, widespread and overall range are immediately apparent. In order to show the shape of data distribution, their central values as well as their variability and box and whisker plots were used for quantitative pollen data (Fig. 3). Largest length of equatorial axis (52.01 μm) was seen in L. mucronatum subsp. orientale thrum, while shortest equatorial axis length (34.86 μm) was found in L. mucronatum subsp. assyriacum thrum. The shortest (42.40 μm) and largest (67.10 μm) polar axis length were found in pin individuals of L. mucronatum subsp. armenum and L. mucronatum subsp. assyriacum respectively. The smallest (0.90) and also the highest (1.86) polar/equatorial axis (P/E) ratio occurred in L. mucronatum subsp. orientale thrum and L. mucronatum subsp. assyriacum thrum respectively. The longest colpi (52.24 μm) were seen in L. mucronatum subsp. assyriacum thrum, while the smallest (31.45 μm) was found in L. mucronatum subsp. armenum pin. ANOVA test showed significant variations (P < 0.01) of some quantitative traits such as equatorial length, colpi width, and apocolpium diameter (Table 3). Pollen sizes in each subspecies varied between thrum and pin individuals, while paired t-test analysis did not show significant variations (P < 0.05) in pollen size within thrum and pin individuals of the studied subspecies.
| Table 3. Results on the ANOVA analysis to assess for differences in quantitative palynological traits in Linum mucronatum subspecies. d.f.: degrees of freedom; F: F-statistic; P: probability. |
|
Characters |
|
Sum of Squares |
d.f. |
Mean Square |
F |
P |
|
Equatorial length |
Between Groups |
4572.329 |
7 |
653.190 |
47.211 |
0.000 |
|
Within Groups |
2656.402 |
192 |
13.835 |
|
|
|
Total |
7228.730 |
199 |
|
|
|
|
Polar length |
Between Groups |
15,639.257 |
7 |
2234.180 |
421.262 |
0.000 |
|
Within Groups |
1018.278 |
192 |
5.304 |
|
|
|
Total |
16,657.536 |
199 |
|
|
|
|
Polar/equatorial axis length ratio |
Between Groups |
22.856 |
7 |
3.265 |
138.328 |
0.000 |
|
Within Groups |
4.532 |
192 |
0.024 |
|
|
|
Total |
27.388 |
199 |
|
|
|
|
Colpi length |
Between Groups |
7430.370 |
7 |
1061.481 |
65.921 |
0.000 |
|
Within Groups |
3091.642 |
192 |
16.102 |
|
|
|
Total |
10,522.013 |
199 |
|
|
|
|
Colpi width |
Between Groups |
2424.905 |
7 |
346.415 |
280.324 |
0.000 |
|
Within Groups |
237.267 |
192 |
1.236 |
|
|
|
Total |
2662.172 |
199 |
|
|
|
|
Colpi length/width ratio |
Between Groups |
4754.569 |
7 |
679.224 |
122.740 |
0.000 |
|
Within Groups |
1062.495 |
192 |
5.534 |
|
|
|
Total |
5817.065 |
199 |
|
|
|
|
Apocolpium
diameter |
Between Groups |
2.082 ×107 |
7 |
2.975×106 |
2.218×106 |
0.000 |
|
Within Groups |
257.456 |
192 |
1.341 |
|
|
|
Total |
2.082 × 107 |
199 |
|
|
|
|
Mesocolpium width |
Between Groups |
2075.442 |
7 |
296.492 |
99.667 |
0.000 |
|
Within Groups |
571.166 |
192 |
2.975 |
|
|
|
Total |
2646.607 |
199 |
|
|
|
|
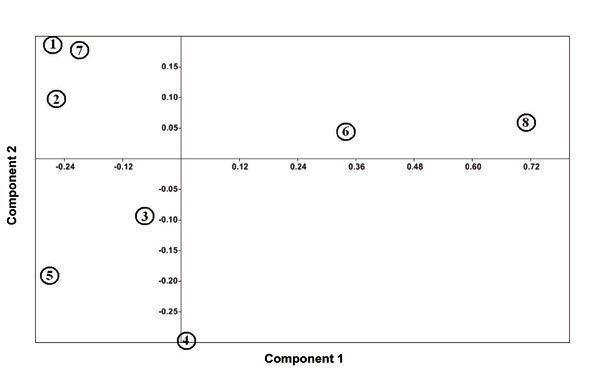
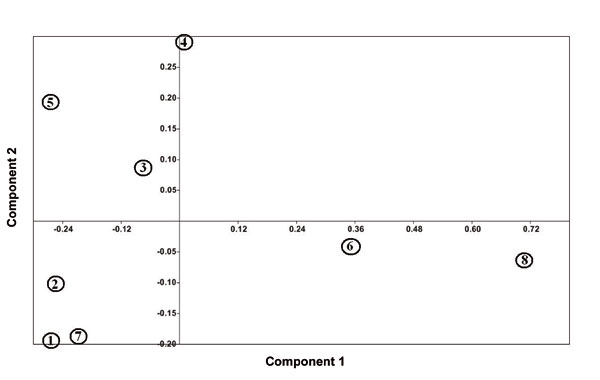
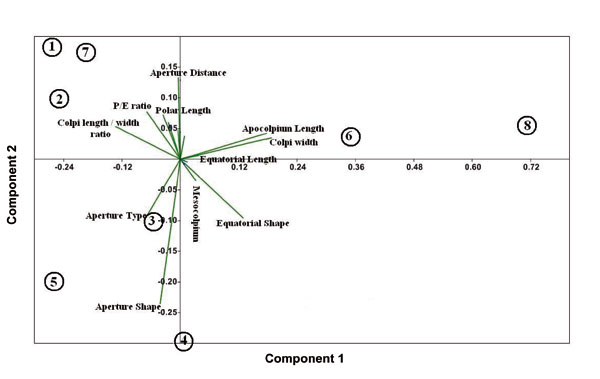
Heterostylous individuals of each subspecies were separated from each other in UPGMA tree (Fig. 4) and also PCA and PCO plots (Figs. 5 and 6). In addition, thrum individuals of L. mucronatum subsp. mucronatum and L. mucronatum subsp. orientale were placed far from others. This subject confirmed variations in pollen features between pin and thrum individuals of each subspecies. PCA biplot showed that some of heterostyled individuals had distinguished traits which became differentiated from others. For example, colpi width and apocolpium diameter were important characters in thrum samples of L. mucronatum subsp. mucronatum and L. mucronatum subsp. orientale or aperture shape was a distinct characteristic of L. mucronatum subsp. armenum thrum (Fig. 7).
|
Figure 5. PCA plot of heterostylous individuals of the studied subspecies: (1), L. mucronatum subsp. assyriacum thrum; (2), L. mucronatum subsp. assyriacum pin; (3), L. mucronatum subsp. armenum pin; (4), L. mucronatum subsp. armenum thrum; (5), L. mucronatum subsp. mucronatum pin; (6), L. mucronatum subsp. mucronatum thrum; (7), L. mucronatum subsp. orientale pin; (8), L. mucronatum subsp. orientale thrum.
[View full size] [Descargar tamaño completo] |
|
|
Figure 6. PCO plot of heterostylous individuals of the studied L. mucronatum subspecies: (1), L. mucronatum subsp. assyriacum thrum; (2), L. mucronatum subsp. assyriacum pin; (3), L. mucronatum subsp. armenum pin; (4), L. mucronatum subsp. armenum thrum; (5), L. mucronatum subsp. mucronatum pin; (6), L. mucronatum subsp. mucronatum thrum; (7), L. mucronatum subsp. orientale pin; (8), L. mucronatum subsp. orientale thrum.
[View full size] [Descargar tamaño completo] |
|
|
Figure 7. PCA biplot of palynological characters of Linum mucronatum subspecies: (1), L. mucronatum subsp. assyriacum thrum; (2), L. mucronatum subsp. assyriacum pin; (3), L. mucronatum subsp. armenum pin; (4), L. mucronatum subsp. armenum thrum; (5), L. mucronatum subsp. mucronatum pin; (6), L. mucronatum subsp. mucronatum thrum; (7), L. mucronatum subsp. orientale pin; (8), L. mucronatum subsp. orientale thrum.
[View full size] [Descargar tamaño completo] |
|
DISCUSSIONTop
Heterostyly is one of the most visible and fascinating examples of convergent evolution in plants, and it is present in all plant forms, from herbs to trees (Vuilleumier, 1967Vuilleumier, B. S. 1967. The origin and evolutionary development of heterostyly in the angiosperms. Evolution 21: 210–226.; Thompson, 2005Thompson, J. D. 2005. Plant Evolution in the Mediterranean. Oxford University Press, New York.). In this study, anther height in thrum flowers was larger than style height; the opposite was observed in pin flowers. In addition to the floral dimorphism, dimorphism in pollen size between thrum and pin flowers has often been reported, thrum pollen grains being larger (e.g. Baker, 1953Baker, H. G. 1953. Dimorphism and monomorphism in the Plumbaginaceae. Annals of Botany 17: 433–445.; Dulberger, 1992Dulberger, R. 1992. Floral polymorphisms and their functional significance in the heterostylous syndrome. In: Barrett, S. C. H. (Ed.), Evolution and function of heterostyly. Springer, Berlin, Heidelberg & New York: 41–84.; Ping & Johnston, 2001Ping, L. & Johnston, M. O. 2001. Comparative floral morphometrics of distyly in three evolutionary lineages of Amsinckia (Boraginaceae). Canadian Journal of Botany 79: 1332–1348.). In the present work, pollen grains traits were examined between pin and thrum individuals of four L. mucronatum subspecies.
Polar shape was similar between pin and thrum individuals of the studied taxa; this characteristic was also fixed among subspecies. In contrast, pollen equatorial shape varied between long and thrum flowers. Equatorial shapes were alike between pin individuals of some subspecies. These conditions were also found in thrum samples of some subspecies (for example, subsp. mucronatum and subsp. armenum); therefore equatorials shapes were analogous between thrum individuals of studied taxa. This has been described in other Linum species; for example, in L. grandiflorum pollen dimorphism was found (Saad, 1961Saad, S. I. 1961. Pollen morphology and sporoderm stratification in Linum. Grana 3: 109–129.; Erdtman, 1964Erdtman, G. 1964. Palynology. In: Turrill, N. B. (Ed.), Vistas in Botany IV. Recent Researches in Plant Taxonomy. Permagon Press, Oxford: 23–54.; Dulberger, 1981Dulberger, R. 1981. Dimorphic exine sculpturing in three distylous species of Linum (Linaceae). Plant Systematics and Evolution 139: 113–119.). The numbers of pollen grains produced per flower and pollen size polymorphism between the morphs are sometimes associated with heterostyly as ancillary characteristics (Naiki, 2012Naiki, A. 2012. Heterostyly and the possibility of its breakdown by polyploidization. Plant Species Biology 27: 3–29.).
In the studied subspecies, pollen size in thrum individuals was larger than in pin ones, with the exception of subsp. assyriacum. This condition was also found in species of the genera Damnacanthus (Naiki & Nagamasu, 2003Naiki, A. & Nagamasu, H. 2003. Distyly and pollen dimorphism in Damnacanthus (Rubiaceae). Journal of Plant Research 116: 105–113.), Plumbago (Ferrero et al., 2009Ferrero, V., de Vega, C., Stafford, G. I., Van Staden, J. & Johnson, S. D. 2009. Heterostyly and pollinators in Plumbago auriculata (Plumbaginaceae). South African Journal of Botany 75: 778–784.) and Polygonum (Chen & Zhang, 2010Chen, M. L. & Zhang, X. P. 2010. Distyly in Polygonum jucundum Meisn. (Polygonaceae). Plant Systematics and Evolution 288: 139–148.).
With the exception of subsp. orientale, pollen surface sculpturing types varied between pin and thrum flowers in each subspecies. Although, the kind of sculpture looked alike in thrum and pin flower in subsp. orientale, the size of surface ornamentation in pollen grains was unequal and presented in small and large parts. In some heterostylous species of Linum such as L. austriacum, L. album and L. glaucum, exine sculpturing structure differed between pin and thrum flowers (Talebi et al., 2012aTalebi, S. M., Sheidai, M., Atri, M., Sharifnia, F. & Noormohammadi, Z. 2012a. Genome size, morphological and palynological variations, and heterostyly in some species of the genus Linum L. (Linaceae) in Iran. African Journal of Biotechnology 11: 16040–16054.). Furthermore dimorphic exine ornamentation was observed in other Linum species for example, L. grandiflorum, L. perenne, L. pubescens, L. mucronatum, L. flavum, and L. maritimum (Dulberger, 1974Dulberger, R. 1974. Structural dimorphism of stigmatic papilla in distylous Linum species. American Journal of Botany 61: 238–243.). Pollen polymorphism associated to the morphs is a typical feature in heterostyly species. Exine pattern of pollen can interact with biotic and abiotic pollination vectors, and it can also interact with the surface area of the stigma interface and mediate stigma adhesions (Talebi et al., 2012aTalebi, S. M., Sheidai, M., Atri, M., Sharifnia, F. & Noormohammadi, Z. 2012a. Genome size, morphological and palynological variations, and heterostyly in some species of the genus Linum L. (Linaceae) in Iran. African Journal of Biotechnology 11: 16040–16054.).
Aperture size, number and complexity affect environmental vulnerability to desiccation, fungal invasion and mechanical stress, and serve as portals for pollen tube exit during germination (Edlund et al., 2004Edlund, A. F., Swanson, R. & Preuss, D. 2004. Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell 16 Suppl: S84–S97.). Wang et al. (2009Wang, H., Yu, W. B., Chen, J. Q. & Blackmore, S. 2009. Pollen morphology in relation to floral types and pollination syndromes in Pedicularis (Orobanchaceae). Plant Systematics and Evolution 277: 153–162.) found that in Pedicularis (Orobanchaceae) there was a significant association between pollen aperture types and corolla types, as well as between pollination syndromes and corolla. There was a distinct correlation between exine ornamentation, floral morphology and pollination in Bauhinia (Ferguson & Pearce, 1986Ferguson, I. K. & Pearce, K. J. 1986. Observations on the pollen morphology of the genus Bauhinia L. (Leguminosae: Caesalpinioideae) in the neotropics. In: Blackmore, S. & Ferguson, I. K. (Eds.), Pollen and spores: form and function. Academic Press, London: 283–296. ). Differences in surface ornamentation in pollen grains have also been reported in some distylous species (Baker, 1953Baker, H. G. 1953. Dimorphism and monomorphism in the Plumbaginaceae. Annals of Botany 17: 433–445., 1956Baker, H. G.1956. Pollen dimorphism in the Rubiaceae. Evolution 10: 23–31.; Dulberger, 1975Dulberger, R. 1975. Intermorph structural differences between stigmatic and pollen grains in relation to incompatibility in Plumbaginaceae. Proceedings of the Royal Society, B, Biological Sciences 188: 257–274.). For example, pollen grains are reticulate or spinulose in Waltheria (Köhler, 1976Köhler, E. 1976. Pollen dimorphism and heterostyly in the genus Waltheria L. (Sterculiaceae). In: Ferguson, I. K. & Muller, J. (Eds), The evolutionary significance of the exine (Linnean Society Symposium, Series 1). Academic Press, London: 147–161.), and smooth or granulate muri in Damnacanthus (Naiki & Nagamasu, 2003Naiki, A. & Nagamasu, H. 2003. Distyly and pollen dimorphism in Damnacanthus (Rubiaceae). Journal of Plant Research 116: 105–113.).
Heterostyly should be considered a morphological variation more than simple variations in anther and style height, because other characters, i.e. morphological and cytological traits show difference between pin and thrum individuals. This condition leads to intra and interpopulation variance. Talebi et al. (2012aTalebi, S. M., Sheidai, M., Atri, M., Sharifnia, F. & Noormohammadi, Z. 2012a. Genome size, morphological and palynological variations, and heterostyly in some species of the genus Linum L. (Linaceae) in Iran. African Journal of Biotechnology 11: 16040–16054.) examined morphological, cytological and also palynological characters of three thrum and pin populations of three species of the genus Linum, namely L. austriacum, L. glaucum and L. album. Results showed a higher mean value of the plant height, size of the basal leaves width, flower leaves width, calyx length, sepal length and petal length occurred in the pin plants, while the mean value of branch number, basal leaves length, flower leaves length, calyx width, pedicel length and sepal length was higher in the thrum plant populations. In their study, t-test analyses of morphological characters showed significant difference for some of the studied characters. Principal coordinate analysis of pin and thrum plant populations based on all morphological characters also separated the two morphs of the three species studied. C-values obtained by flow cytometry, differed in the pin and thrum plants of the studied species and also the aperture shape differed between these populations (Talebi et al., 2012aTalebi, S. M., Sheidai, M., Atri, M., Sharifnia, F. & Noormohammadi, Z. 2012a. Genome size, morphological and palynological variations, and heterostyly in some species of the genus Linum L. (Linaceae) in Iran. African Journal of Biotechnology 11: 16040–16054.).
The arrangements of pin and thrum individuals of the studied taxa in plots and tree were very interesting as the heterostylous individuals of some subspecies were separated from each other. For example, thrum individuals of subsp. orientale and subsp. mucronatum were closely tougher.
Similarities in pollen can influence palynological taxonomic treatment in this genus. Pollen morphology of the genus Linum could not firmed any of the previously proposed arranging the species in sections (Grigoryevka, 1988Grigoryevka, V. V. 1988. [The pollen grain morphology in the genus Linum (Linaceae) of the flora of the USSR]. Botanicheskii Zhurnal SSSR 73: 1409–1417 [in Russian].) and also the obtained palynological data of fifteen Linum taxa cannot show the species relationship in sections and also infraspecific classification in the mentioned genus (Talebi et al., 2012bTalebi, S. M., Sheidai, M., Atri, M., Sharifnia, F. & Noormohammadi, Z. 2012b. Palynological study of the genus Linum in Iran (a taxonomic review). Phytologia Balcanica 18: 293–303. ).The reasons of these uncongenial may be related to heterostyly phenomenon which abundantly present in this genus and about 40% of these species are distylous and occurred in four out of five sections of the genus, namely Linum, Syllinum, Dasylinum and Linastrum (Rogers, 1979Rogers, C. M. 1979. Distyly and pollen dimorphism in Linum suffruticosum (Linaceae). Plant Systematics and Evolution 131: 127–132.; Sharifnia & Assadi, 2001Sharifnia, F. & Assadi, M. 2001. Flora of Iran, No. 34: Linaceae. Research Institute of Forests (Ministry of Jahad-e-Sazandegi and Rangelands, Islamic Republic of Iran), Tehran.).
Two major models were considered for evolution of heterostyly (mainly distyly) in plants: change in flowers morphology or diallelic self-incompatibility designing by Charlesworth & Charlesworth (1979Charlesworth, D. & Charlesworth, B. 1979. A model for the evolution of distyly. American Naturalist 114: 467–498.). Based on this model, under conditions of pollinator limitation, self-incompatibility first occurred within a homostylous population as a result of selection to prevent self-fertilization then stigma height polymorphism occurred to reduce intra-flower interference between males and females, and subsequently reciprocal herkogamy evolved to promote legitimate pollination. Another model proposed by Lloyd & Webb (1992Lloyd, D. G. & Webb, C. J. 1992. The evolution of heterostyly. In: Barrett, S. C. H. (Ed.), Evolution and function of heterostyly. Springer, Berlin: 151–178.). These authors proposed that the ancestral flowers showed approach herkogamy, and subsequent polymorphism in stigma height followed by invading and spreading a mutant that had shortened style length, and then reciprocal herkogamy was established by the appearance of reverse herkogamous mutants. Heteromorphic self-incompatibility may arise if selection restricts self-fertilization. The phylogenetic study of Linum and Linaceae subfamily Linoideae indicates that neither heterostyly nor homostyly can yet be confirmed as the ancestral state in Linoideae or Linaceae, but provide strong evidence that breeding system is evolutionarily labile in this group (McDill et al., 2009McDill, J., Repplinger, M., Simpson, B. B. & Kadereit, J. W. 2009.The phylogeny of Linum and Linaceae subfamily Linoideae, with implications for their systematics, biogeography and evolution of heterostyly. Systematic Botany 34: 386-405.).
REFERENCESTop
|
| 1. | APG III. 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161: 105–121. |
|
| 2. | Armbruster, W. S., Pérez-Barrales, R., Arroyo, J., Edwards, M. E. & Vargas, P. 2006. Three-dimensional reciprocity of floral morphs in wild flax (Linum suffruticosum): a new twist on heterostyly. New Phytologist 171: 581–590. |
|
| 3. | Baker, H. G. 1953. Dimorphism and monomorphism in the Plumbaginaceae. Annals of Botany 17: 433–445. |
|
| 4. | Baker, H. G.1956. Pollen dimorphism in the Rubiaceae. Evolution 10: 23–31. |
|
| 5. | Barrett, S. C. H. 1992. Evolution and function of heterostyly. Springer, Berlin. |
|
| 6. | Barrett, S. C. H. 2010. Darwin’s legacy: the forms, function and sexual diversity of flowers. Philosophical Transactions of the Royal Society of London, B, Biological Sciences 365: 351–368. |
|
| 7. | Barrett, S. C. H. & Shore, J. S. 2008. New insights on heterostyly: Comparative biology, ecology and genetics. In: Franklin-Tong, V. (Ed.), Self-incompatibility in flowering plants: Evolution, diversity and mechanisms. Springer-Verlag, Berlin: 3–32 |
|
| 8. | Charlesworth, D. & Charlesworth, B. 1979. A model for the evolution of distyly. American Naturalist 114: 467–498. |
|
| 9. | Chen, M. L. & Zhang, X. P. 2010. Distyly in Polygonum jucundum Meisn. (Polygonaceae). Plant Systematics and Evolution 288: 139–148. |
|
| 10. | Cohen, J. I. 2010. ‘A case to which no parallel exists’: the influence of Darwin’s different forms of flowers. American Journal of Botany 97: 701–716. |
|
| 11. | Darwin, C. 1877. The different forms of flowers on plants of the same species. John Murray, London. |
|
| 12. | Dulberger, R. 1973. Distyly in Linum pubescens and L. mucronatum. Botanical Journal of the Linnean Society 66: 117–126. |
|
| 13. | Dulberger, R. 1974. Structural dimorphism of stigmatic papilla in distylous Linum species. American Journal of Botany 61: 238–243. |
|
| 14. | Dulberger, R. 1975. Intermorph structural differences between stigmatic and pollen grains in relation to incompatibility in Plumbaginaceae. Proceedings of the Royal Society, B, Biological Sciences 188: 257–274. |
|
| 15. | Dulberger, R. 1981. Dimorphic exine sculpturing in three distylous species of Linum (Linaceae). Plant Systematics and Evolution 139: 113–119. |
|
| 16. | Dulberger, R. 1992. Floral polymorphisms and their functional significance in the heterostylous syndrome. In: Barrett, S. C. H. (Ed.), Evolution and function of heterostyly. Springer, Berlin, Heidelberg & New York: 41–84. |
|
| 17. | Edlund, A. F., Swanson, R. & Preuss, D. 2004. Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell 16 Suppl: S84–S97. |
|
| 18. | Erdtman, G. 1960. The acetolysis method. Svensk Botanisk Tidskrift 54: 561–564. |
|
| 19. | Erdtman, G. 1964. Palynology. In: Turrill, N. B. (Ed.), Vistas in Botany IV. Recent Researches in Plant Taxonomy. Permagon Press, Oxford: 23–54. |
|
| 20. | Ferguson, I. K. & Pearce, K. J. 1986. Observations on the pollen morphology of the genus Bauhinia L. (Leguminosae: Caesalpinioideae) in the neotropics. In: Blackmore, S. & Ferguson, I. K. (Eds.), Pollen and spores: form and function. Academic Press, London: 283–296. |
|
| 21. | Ferrero, V., de Vega, C., Stafford, G. I., Van Staden, J. & Johnson, S. D. 2009. Heterostyly and pollinators in Plumbago auriculata (Plumbaginaceae). South African Journal of Botany 75: 778–784. |
|
| 22. | Ganders, F. R. 1979. The biology of heterostyly. New Zealand Journal of Botany 17: 607–635. |
|
| 23. | Grigoryevka, V. V. 1988. [The pollen grain morphology in the genus Linum (Linaceae) of the flora of the USSR]. Botanicheskii Zhurnal SSSR 73: 1409–1417 [in Russian]. |
|
| 24. | Güvensen, A., Seçmen, Ö. & Şenol, S. G. 2013. Heterostyly in Linum aretioides. Turkish Journal of Botany 37: 122–129. |
|
| 25. | Heywood, V. H. 1993. Flowering plants of the world. Oxford University Press, Oxford. |
|
| 26. | Köhler, E. 1976. Pollen dimorphism and heterostyly in the genus Waltheria L. (Sterculiaceae). In: Ferguson, I. K. & Muller, J. (Eds), The evolutionary significance of the exine (Linnean Society Symposium, Series 1). Academic Press, London: 147–161. |
|
| 27. | Lloyd, D. G. & Webb, C. J. 1992. The evolution of heterostyly. In: Barrett, S. C. H. (Ed.), Evolution and function of heterostyly. Springer, Berlin: 151–178. |
|
| 28. | McDill, J., Repplinger, M., Simpson, B. B. & Kadereit, J. W. 2009.The phylogeny of Linum and Linaceae subfamily Linoideae, with implications for their systematics, biogeography and evolution of heterostyly. Systematic Botany 34: 386-405. |
|
| 29. | Moore, P. D., Webb, J. A. & Collinson, M. E. 1991. Pollen analysis (2nd ed.). Blackwell Scientific Publications, Oxford. |
|
| 30. | Naiki, A. 2012. Heterostyly and the possibility of its breakdown by polyploidization. Plant Species Biology 27: 3–29. |
|
| 31. | Naiki, A. & Nagamasu, H. 2003. Distyly and pollen dimorphism in Damnacanthus (Rubiaceae). Journal of Plant Research 116: 105–113. |
|
| 32. | Ockendon, D. G. 1968. Biosystematic studies. In: Linum perenne group. New Phytologist 67: 787–813. |
|
| 33. | Ockendon, D. J. & Walters, S. M. 1968. Linum L. In: Tutin, T. G., & Heywood, V. H. (Eds.), Flora Europaea 2. Cambridge University Press, Cambridge: 206–211. |
|
| 34. | Özcan, T. & Zorlu, E. 2009. A contribution to taxonomy of Turkish Linum based on seed surface patterns. Biologia (Bratislava), Section Botany 64: 723–730. |
|
| 35. | Pérez-Barrales, R., Vargas, P. & Arroyo, J. 2006. New evidence for the Darwinian hypothesis of heterostyly: breeding systems and pollinators in Narcissus sect. Apodanthi. New Phytologist 171: 553–567. |
|
| 36. | Ping, L. & Johnston, M. O. 2001. Comparative floral morphometrics of distyly in three evolutionary lineages of Amsinckia (Boraginaceae). Canadian Journal of Botany 79: 1332–1348. |
|
| 37. | Podani, J. 2000. Introduction to the exploration of multivariate biological data. Backhuys Publishers, Leiden. |
|
| 38. | Richards, A. J. 1997. Plant breeding systems (2nd ed.). Chapman and Hall, London. |
|
| 39. | Richards, J. H. & Barrett, S. C. H. 1992. The development of heterostyly. In: Barrett, S. C. H. (Ed.), Evolution and function of heterostyly. Springer, Berlin, Heidelberg & New York: 85–127. |
|
| 40. | Rogers, C. M. 1979. Distyly and pollen dimorphism in Linum suffruticosum (Linaceae). Plant Systematics and Evolution 131: 127–132. |
|
| 41. | Rohlf, F. J. 1998. NTSYS-PC. Numerical taxonomy and multivariate analysis system. version 2.02i. Applied Biostatistics, Inc., New York. |
|
| 42. | Saad, S. I. 1961. Pollen morphology and sporoderm stratification in Linum. Grana 3: 109–129. |
|
| 43. | Sharifnia, F. & Assadi, M. 2001. Flora of Iran, No. 34: Linaceae. Research Institute of Forests (Ministry of Jahad-e-Sazandegi and Rangelands, Islamic Republic of Iran), Tehran. |
|
| 44. | Talebi, S. M., Sheidai, M., Atri, M., Sharifnia, F. & Noormohammadi, Z. 2012a. Genome size, morphological and palynological variations, and heterostyly in some species of the genus Linum L. (Linaceae) in Iran. African Journal of Biotechnology 11: 16040–16054. |
|
| 45. | Talebi, S. M., Sheidai, M., Atri, M., Sharifnia, F. & Noormohammadi, Z. 2012b. Palynological study of the genus Linum in Iran (a taxonomic review). Phytologia Balcanica 18: 293–303. |
|
| 46. | Thompson, J. D. 2005. Plant Evolution in the Mediterranean. Oxford University Press, New York. |
|
| 47. | Vuilleumier, B. S. 1967. The origin and evolutionary development of heterostyly in the angiosperms. Evolution 21: 210–226. |
|
| 48. | Wang, H., Yu, W. B., Chen, J. Q. & Blackmore, S. 2009. Pollen morphology in relation to floral types and pollination syndromes in Pedicularis (Orobanchaceae). Plant Systematics and Evolution 277: 153–162. |
|
| 49. | Weller, S. G. 2009. The different forms of flowers—what have we learned since Darwin? Botanical Journal of the Linnean Society 160: 249–261. |
|
| 50. | Xavier, K. S. & Rogers, C. M. 1963. Pollen morphology as a taxonomic tool in Linum. Rhodora 65: 137–145. |