ARTÍCULO
Re-evaluation of the Helichrysum italicum complex (Compositae: Gnaphalieae): A new species from Majorca (Balearic Islands)
S. HERRANDO-MORAIRA1, J. M. BLANCO-MORENO2, L. SÁEZ1,3 & M. GALBANY-CASALS1
1 Departament de Biologia Animal, Biologia Vegetal i Ecologia, Facultat de Biociències, Universitat Autònoma de Barcelona, ES-08193 Bellaterra, Spain
2 Departament de Biologia Vegetal & IRBio Universitat de Barcelona, av. Diagonal, 643, ES-08028 Barcelona, Spain
3 Societat d’Història Natural de les Illes Balears (SHNB), c. Margarida Xirgu, 16, ES-07003 Palma de Mallorca, Balearic Islands, Spain
Author for correspondence: S. Herrando-Moraira (sonia.herrando@gmail.com)
Editor: N. Garcia-Jacas
ABSTRACT
Re-evaluation of the Helichrysum italicum complex (Compositae: Gnaphalieae): A new species from Majorca (Balearic Islands).— Helichrysum italicum is a widely distributed species in the Mediterranean Basin, which has received controversial taxonomic treatments at infraspecific level owing to its morphological variability. In this paper we perform a detailed multivariate analysis of morphological characters using an exhaustive sampling of H. italicum. Integrating previously published molecular, chorological and newly obtained morphological data, a revised taxonomic treatment for the whole H. italicum complex is provided. On the one hand, the results obtained suggest that the Majorcan mountain populations of H. italicum subsp. microphyllum need to be considered an independent species, newly described here as Helichrysum massanellanum. On the other hand, H. italicum subspecies are recircumscribed. A new combination is proposed, H. italicum subsp. tyrrhenicum, a taxon that comprises populations from Corsica, Sardinia, Majorca coastline and Dragonera islet, whereas subsp. microphyllum is restricted to the island of Crete. With those substantial classification modifications, a complete new taxonomic treatment is presented for this group, including an identification key, synonyms, morphological descriptions, distribution areas and habitat characterization.
KEY WORDS: discriminant analysis; endemism; Mediterranean Basin; morphometrics; principal component analysis; taxonomy.
Reevaluación del complejo Helichrysum italicum (Compositae: Gnaphalieae): una nueva especie en Mallorca (Islas Baleares)
RESUMEN
Reevaluación del complejo Helichrysum italicum (Compositae: Gnaphalieae): una nueva especie en Mallorca (Islas Baleares).— Helichrysum italicum es una especie con una amplia distribución en la cuenca Mediterránea, la cual ha recibido tratamientos taxonómicos controvertidos a nivel infraespecifico debido a su variabilidad morfológica. En este trabajo realizamos un detallado análisis multivariante de los caracteres morfológicos sobre la base de un muestreo exhaustivo de H. italicum. Integrando datos moleculares y corológicos previamente publicados con los datos morfológicos obtenidos, se presenta una revisión del tratamiento taxonómico para todo el complejo H. italicum. Por un lado, los resultados obtenidos sugieren que las poblaciones de H. italicum subsp. microphyllum localizadas en la montaña de Mallorca deben ser consideradas como una especie independiente, descrita aquí como Helichrysum massanellanum. Por otro lado, las subespecies de H. italicum son recircunscritas. Se propone una nueva combinación, H. italicum subsp. tyrrhenicum, un taxón que comprende poblaciones de Córcega, Cerdeña, la costa de Mallorca y el islote de Dragonera, mientras que la subsp. microphyllum queda restringida a la isla de Creta. Con estas modificaciones sustanciales, se presenta un tratamiento taxonómico completo y nuevo para este grupo, incluyendo una clave de identificación, sinónimos, descripciones morfológicas, áreas de distribución y caracterización del hábitat.
PALABRAS CLAVE: análisis de componentes principales; análisis discriminante; cuenca Mediterránea; endemismo; morfometría; taxonomía.
Recibido: 10/11/2015 / Aceptado: 12/01/2016 / Publicado on line: 01/09/2016
Cómo citar este artículo / Citation: Herrando-Moraira, S., Blanco-Moreno, J. M., Sáez, L. & Galbany-Casals, M. 2016. Re-evaluation of the Helichrysum italicum complex (Compositae: Gnaphalieae): A new species from Majorca (Balearic Islands). Collectanea Botanica 35: e009. doi: http://dx.doi.org/10.3989/collectbot.2016.v35.009
Copyright: © 2016 Institut Botànic de Barcelona (CSIC). This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) Spain 3.0.
CONTENIDOS
INTRODUCTIONTop
The flora of the Mediterranean Basin is characterized by very high species diversity and high levels of endemicity; it encompasses ca. 25,000 species, of which 13,000 are endemics (Myers et al., 2000Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A. & Kent, J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858.http://dx.doi.org/10.1038/35002501). In particular, the highest biodiversity and highest level of endemism are concentrated in the 10 hot-spots recognized by Médail & Quézel (1997Médail, F. & Quézel, P. 1997. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean Basin. Annals of the Missouri Botanical Garden 84: 112–127. http://dx.doi.org/10.2307/2399957). In the context of the Western Mediterranean area, the Balearic Islands are one of these biodiversity hotspots. This archipelago has also been considered a biodiversity refuge (Médail & Diadema, 2009Médail, F. & Diadema, K. 2009. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. Journal of Biogeography 36: 1333–1345. http://dx.doi.org/10.1111/j.1365-2699.2008.02051.x) and is relatively rich in terms of vascular plants diversity and endemism. Recent data (Sáez et al., 2013Sáez, L., Fraga, P. & López-Alvarado, J. 2013. The flora of the Balearic Islands. In: Cardona, E., Estaún, I., Comas, M. & Fraga, P. (Eds.), Islands and plants: preservation and understanding of flora on Mediterranean Islands. Consell Insular de Menorca, Maó: 91–103.) suggest that the Balearic Islands harbour 1551 taxa, 140 of which are endemic (9%). Moreover, nearly 36% of the endangered (IUCN, 2001IUCN (International Union for Conservation of Nature) 2001. IUCN Red List categories and criteria Version 3.1. IUCN Species Survival Commission, Gland,& Cambridge.) Balearic taxa are endemisms.
The genus Helichrysum Mill. (Compositae, Gnaphalieae) is distributed throughout the African continent, Madagascar, the Mediterranean Basin, Macaronesia, Middle Asia and India (Anderberg, 1991Anderberg, A. A. 1991. Taxonomy and phylogeny of the tribe Gnaphalieae (Asteraceae). Opera Botanica 104: 1–195.), and comprises ca. 500 (Hilliard, 1983Hilliard, O. M. 1983. Helichrysum Mill. In: Leistner, O. A. (Ed.), Flora of Southern Africa 33 (7, 2). Department of Agriculture, Pretoria: 61–310. ) to ca. 600 (Anderberg, 1991Anderberg, A. A. 1991. Taxonomy and phylogeny of the tribe Gnaphalieae (Asteraceae). Opera Botanica 104: 1–195.) species. Section Stoechadina (DC.) Gren. & Godr. comprises eight Mediterranean species (Galbany-Casals et al., 2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082), and five of them are present in the Balearic Islands. This section is well supported as monophyletic in phylogenies based on nuclear ribosomal DNA sequences (Galbany-Casals et al., 2009Galbany-Casals, M., Garcia-Jacas, N., Sáez, L., Benedí, C. & Susanna, A. 2009. Systematics, biogeography and character evolution in Mediterranean, Asiatic and Macaronesian Helichrysum (Asteraceae, Gnaphalieae) inferred from nuclear phylogenetic analyses. International Journal of Plant Sciences 170: 365–380. http://dx.doi.org/10.1086/596332) and contains a subclade, called the Helichrysum italicum complex, which comprises three species sharing two morphological characters: cylindrical to cylindrical-campanulate capitula and outermost involucral bracts partially or completely herbaceous and covered with a dense indumentum. These species are Helichrysum serotinum (DC.) Boiss., with two subspecies—H. serotinum subsp. serotinum and H. serotinum subsp. picardii (Boiss. & Reut.) Galbany, L. Sáez & Benedí—, Helichrysum litoreum Guss. and Helichrysum italicum (Roth) G. Don (Galbany-Casals et al., 2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082). A recent taxonomic treatment (Galbany-Casals et al., 2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082) recognised three subspecies within H. italicum: Helichrysum italicum subsp. italicum has the widest distribution and is found from the westernmost isolated localities in Morocco to the eastermost localities in Cyprus, being very common particularly in Italy, Corsica, and some Aegean Islands; H. italicum subsp. siculum (Jord. & Fourr.) Galbany, L. Sáez & Benedí is endemic to Sicily; and H. italicum subsp. microphyllum (Willd.) Nyman was originally described from Crete (Willdenow, 1803Willdenow, C. L. 1803. Caroli a Linné Species Plantarum [...] editio quarta [...], 3(3). Impensis G. C. Nauk, Berolini.) and has a disjunct distribution in the Balearic Islands (Majorca and Dragonera), Corsica, Sardinia, Crete and Cyprus (Galbany-Casals et al., 2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082).
The identity of the Balearic plants belonging to H. italicum has been rather controversial. The first record of H. italicum subsp. microphyllum from the Balearic Islands (Cambessèdes, 1827Cambessèdes, J. 1827. Enumeratio plantarum quas in insulis Balearibus collegit J. Cambèssedes. Mémoires du Muséum d’Histoire Naturelle. Paris 14: 173–335., sub H. microphyllum) corresponds to a high mountain locality of Serra de Tramuntana (northern Majorca). Later, this taxon was also recorded from coastal areas of western Majorca and from Dragonera, an islet located ca. 1 km off the coast of western Majorca (Marès & Vigineix, 1880Marès, P. & Vigineix, G. 1880. Catalogue raisonné des plantes vasculaires des îles Baléares. Ed. G. Masson, Paris.; Knoche, 1922Knoche, H. 1922. Flora Balearica. Etude phytogéographique sur les îles Baléares 2. Imp. Roumégous et Déhen, Montpellier.; Duvigneaud, 1979Duvigneaud, J. 1979. Catalogue provisoire de la flore des Baléares (2nd ed.). Bulletin de la Société pour l’Échange des Plantes Vasculaires de l’Europe et du Bassin Méditerranéen 17: 1–43.; Bonafè, 1980Bonafè, F. 1980. Flora de Mallorca 4. Ed. Moll, Palma de Mallorca.).
Some authors suggested that the plants from the coastal localities and the plants from the mountain localities in Majorca present some differences. Pla et al. (1992Pla, V., Sastre, B. & Llorens, L. 1992. Aproximació al catàleg de la flora vascular de les illes Balears: 1992. Universitat de les Illes Balears, Palma.) suggested the existence of two subspecies of H. italicum within Majorcan populations: subsp. microphyllum and another unnamed subspecies, which was considered to be endemic to the island of Majorca but without indication of a particular locality. Sáez & Rosselló (2001Sáez, L. & Rosselló, J. A. 2001. Llibre Vermell de la flora amenaçada de les IIles Balears. Conselleria de Medi Ambient (Govern de les Illes Balears), Palma de Mallorca.) also considered that populations restricted to mountain areas of northern Majorca would be assignable to Helichrysum microphyllum (Willd.) Cambess.—at the species level—, whereas coastal populations from western Majorca and Dragonera islet would not correspond to typical H. microphyllum, but they did not suggest any alternative taxonomic treatment for them. Later, Angiolini et al. (2005Angiolini, C., Bacchetta, G., Brullo, S., Casti, M., Giusso del Galdo, G. & Guarino, R. 2005. The vegetation of mining dumps in SW-Sardinia. Feddes Repertorium 116: 243–276. http://dx.doi.org/10.1002/fedr.200411072) also accepted H. microphyllum at the species level, but considered that the populations from Crete differ from the western Mediterranean populations, and the latter were called H. microphyllum subsp. tyrrhenicum Bacch., Brullo & Giusso. This taxon included populations from Corsica, Sardinia and the Balearic Islands, although these authors did not make distinction between the coastal and mountain populations of Majorca. In contrast, Jeanmonod (1998Jeanmonod, D. 1998. Xanthium subg. Xanthium et Helichrysum italicum deux cas taxonomiques ardus. Candollea 53: 435–457.) found greater similarities between populations from Crete and Majorca than between any of them and populations from Corsica, but he did not study the coastal populations of Majorca. Finally, Galbany-Casals et al. (2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082) indicated the existence of some morphological differences between mountain and coastal populations from Majorca, but these authors included both groups of populations in their broad concept of H. italicum subsp. microphyllum, which also comprised populations from Corsica, Sardinia, Crete and Cyprus.
The identity of the Cypriot plants has also been discussed in the literature. Boissier (1840Boissier, E. 1840. Voyage botanique dans le midi de l’Espagne pendant l’année 1837 2. Gide et Cie, Paris.) initially considered them to belong to the current concept of H. italicum subsp. italicum (sub H. serotinum var. orientale Boiss.), although later he included them in a broad concept of H. italicum subsp. microphyllum (Boissier, 1875Boissier, E. 1875. Flora orientalis sive enumeratio plantarum in Oriente a Graecia et Aegypto and Indiae fines hucusque observatarum 3. H. Georg, Basileae & Genevae., sub H. italicum β microphyllum Boiss.). Holmboe (1914Holmboe, J. 1914. Studies on the vegetation of Cyprus: Based upon researches during the spring and summer 1905. John Griegs, Bergen.) described H. italicum var. canum to accommodate the Cypriot plants, which was said to be chiefly recognized by their numerous small axillary branches, short dense-sitting leaves and intensely tomentose involucres. Georgiadou (1985Georgiadou, E. 1985. Helichrysum. In: Meikle, R. D. (Ed.), Flora of Cyprus 2. Royal Botanic Gardens, Kew: 888–889.) considered the Cypriot plants under H. italicum, whereas Galbany-Casals et al. (2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082) recognized the existence of two subspecies of H. italicum in Cyprus, subsp. italicum and subsp. microphyllum. Finally, Greuter (2008Greuter, W. 2008. Helichrysum Mill. In: Greuter, W. & Raab-Straube, E. von (Eds.), Med-checklist 2 Dicotyledones (Compositae). Organisation for the Phyto-Taxonomic Investigation of the Mediterranean Area (OPTIMA), Genève: 234–239.) recognised the Cypriot populations under H. italicum subsp. italicum.
Although H. italicum subsp. microphyllum is reasonably uniform in the diagnostic characters that separate it from subsp. italicum—short leaves, presence of abundant axillary fascicles and relatively short height—it is quite variable in other aspects of its morphology, which has motivated the controversy in its taxonomic classification, as detailed above. The morphological variation between populations from several islands, together with its disjunct distribution area, and the morphological transition of this subspecies to subsp. italicum particularly in Corsica but also in the eastern Mediterranean, motivated an integrated morphological and molecular study focused on H. italicum and the origin of subsp. microphyllum (Galbany-Casals et al., 2011Galbany-Casals, M., Blanco-Moreno, J. M., Garcia-Jacas, N., Breitwieser, I. & Smissen, R. D. 2011. Genetic and morphological variation in the Mediterranean Helichrysum italicum subsp. microphyllum (Asteraceae; Gnaphalieae). Plant Biology 13: 678–687. http://dx.doi.org/10.1111/j.1438-8677.2010.00411.x). Based on AFLP and plastid sequences as well as morphological characters, these authors suggested that subsp. microphyllum and subsp. italicum morphologies are the product of local selection acting on a common gene pool, as strong genetic barriers do not exist between them. Helichrysum italicum s. l. was found to be structured in three main genetic groups, corresponding to geographic divisions in the Mediterranean Basin: a western, a central and an eastern group. Therefore, the character syndrome corresponding with subsp. microphyllum would have arisen within the wider H. italicum gene pool independently in the eastern Mediterranean (Crete) and in the western Mediterranean (Corsica, Sardinia and Balearic Islands), and in a similar way subsp. siculum would have arisen within H. italicum in the Central Mediterranean (Sicily). However, this work was based on a limited set of morphological characters and was particularly focused on the morphological variation found in Corsica and the gene flow between subsp. italicum and subsp. microphyllum in that island, whereas morphological variation found within Majorca was not studied in detail.
In this paper we perform a multivariate analysis of morphological characters using an exhaustive sampling of H. italicum with the aim of evaluating the taxonomic identity of the Majorcan mountain populations of H. italicum subsp. microphyllum. In the light of the results obtained, we also provide a revised taxonomic treatment for the whole H. italicum complex.
MATERIALS AND METHODSTop
Morphological variation of H. italicum was evaluated through 33 characters recognised as taxonomically relevant (Table 1), which were chosen on the basis of previous morphometric studies (Galbany-Casals et al., 2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082, 2011Galbany-Casals, M., Blanco-Moreno, J. M., Garcia-Jacas, N., Breitwieser, I. & Smissen, R. D. 2011. Genetic and morphological variation in the Mediterranean Helichrysum italicum subsp. microphyllum (Asteraceae; Gnaphalieae). Plant Biology 13: 678–687. http://dx.doi.org/10.1111/j.1438-8677.2010.00411.x) or that appeared to be variable in the course of the present study (S. Herrando, pers. obs.). Of the total 33 characters studied, 23 were quantitative, eight were semiquantitative and two were qualitative (Table 1).
| Table 1. Morphological variables used in morphometric analyses. |
| Morphological variables |
Type |
|
Vegetative characters |
|
Presence (1) / absence (0) of axillary leaf fascicles
|
Qualitative
|
|
Caulinar leaf length (mm)
|
Quantitative
|
|
Caulinar leaf width (mm)
|
Quantitative
|
|
Caulinar leaf length / caulinar leaf width
|
Quantitative
|
|
Leaf margin (flat or revolute) (0–4)1
|
Semiquantitative
|
|
Leaf margin undulation (0–1)2
|
Qualitative
|
|
Eglandular indumentum of leaf adaxial side (0–4)3
|
Semiquantitative
|
|
Glandular indumentum of leaf abaxial side (0–4)3
|
Semiquantitative
|
|
Floral characters |
|
Synflorescence length (mm)
|
Quantitative
|
|
Synflorescence width (mm)
|
Quantitative
|
|
Number of capitula per synflorescence
|
Quantitative
|
|
Capitulum length (mm)
|
Quantitative
|
|
Capitulum width (mm)
|
Quantitative
|
|
Capitulum length / capitulum width
|
Quantitative
|
|
Number of hermaphroditic florets per capitulum
|
Quantitative
|
|
Number of pistillate florets per capitulum
|
Quantitative
|
|
Total number of florets per capitulum
|
Quantitative
|
|
Outermost involucral bract length (mm)
|
Quantitative
|
|
Outermost involucral bract width (mm)
|
Quantitative
|
|
Outermost involucral bract length / outermost involucral bract width
|
Quantitative
|
|
Outermost involucral bract texture (0–1)4
|
Semiquantitative
|
|
Eglandular indumentum of outermost involucral bract (0–4)3
|
Semiquantitative
|
|
Glandular indumentum of outermost involucral bract (0–4)3
|
Semiquantitative
|
|
Innermost involucral bract length (mm)
|
Quantitative
|
|
Innermost involucral bract width (mm)
|
Quantitative
|
|
Innermost involucral bract length / innermost involucral bract width
|
Quantitative
|
|
Eglandular indumentum of innermost involucral bract (0–4)3
|
Semiquantitative
|
|
Glandular indumentum of innermost involucral bract (0–4)3
|
Semiquantitative
|
|
Average of innermost involucral bract length / outermost involucral bract length
|
Quantitative
|
|
Number of involucral bracts per capitulum
|
Quantitative
|
|
Hermaphroditic florets length
|
Quantitative
|
|
Pistillate florets length
|
Quantitative
|
|
Pappus setae length
|
Quantitative
|
1 0: all leaves flat; 1: most leaves flat, some revolute; 2: flat and revolute leaves in the same proportion; 3: most leaves revolute, some flat; 4: all leaves revolute.
2 0: most leaves without undulate margins; 0.5: some leaves undulate; 1: most leaves with undulate margins.
3 0: 0–5% coverage; 1: 6–25% coverage; 2: 26–50% coverage; 3: 51–75% coverage; 4: 76–100% coverage.
4 0: bract totally papery; 0.5: bract herbaceous in its basal half and papery in its distal half; 1: bract totally herbaceous. |
|
Features of involucral bracts, florets and indumentum were studied under a ZEISS Stemi DV4 binocular stereoscopic microscope. For the quantitative characters, the mean of three measurements per specimen was used in the analysis when possible. Owing to the perceived differences in size and shape of some characters among the different taxa, the quantitative characters comprise direct measurements (18 characters) as well as ratios between some of them (five characters) (Table 1). An additional qualitative character, the orientation of caulinar leaves, was also recorded. However, this character was only available for a subset of the specimens and thus excluded from final analyses. Terminology and description of characters follow Galbany-Casals et al. (2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082).
A total of 119 specimens from 65 populations were used for morphometric multivariate analyses (Table 2; see Appendix 1 for the list of all specimens examined and included in the analyses). Sampling was based on our own field collections and additional herbarium material with the aim to cover the entire distribution area of the studied taxa and to encompass their overall morphological variation. Taxa or specimens of hypothesised hybrid origin, such as Helichrysum pseudolitoreum (Fiori) Brullo (Galbany-Casals et al., 2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082) or some coastal populations from western Majorca with intermediate morphological appearance between H. italicum subsp. microphyllum and Helichrysum stoechas (L.) Moench, were deliberately excluded from this study. An exploratory Principal Component Analysis (PCA) based on the correlation between characters was performed with SPSS v17.0 (SPSS Inc., Chicago, IL, USA) using individuals as Operational Taxonomic Units. PCA was used to reduce the overall variation of the morphological characters into new uncorrelated components to explore the pattern of morphological variation among individuals. Three characters—lengths of hermaphroditic florets, of pistillate florets and of pappus setae—were not included in this analysis because data were not available for all individuals. The aim of this analysis was to evaluate the morphological variation within H. italicum, as well as to analyze the cohesion of previously recognized taxa and the strength of their morphological affinity. To ease the evaluation of the morphological congruence of the existing taxonomic treatments with the PCA, the individuals were labeled in the scatterplots according to predefined groups. First we considered the three groups accepted in Galbany-Casals et al. (2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082): (1) H. italicum subsp. italicum, (2) H. italicum subsp. microphyllum and (3) H. italicum subsp. siculum. Then they were labelled considering six groups, which were established to integrate together H. italicum genetic structure as well as the morphological variation reported in previous works (Sáez & Rosselló, 2001Sáez, L. & Rosselló, J. A. 2001. Llibre Vermell de la flora amenaçada de les IIles Balears. Conselleria de Medi Ambient (Govern de les Illes Balears), Palma de Mallorca.; Angiolini et al., 2005Angiolini, C., Bacchetta, G., Brullo, S., Casti, M., Giusso del Galdo, G. & Guarino, R. 2005. The vegetation of mining dumps in SW-Sardinia. Feddes Repertorium 116: 243–276. http://dx.doi.org/10.1002/fedr.200411072; Galbany-Casals et al., 2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082, 2011Galbany-Casals, M., Blanco-Moreno, J. M., Garcia-Jacas, N., Breitwieser, I. & Smissen, R. D. 2011. Genetic and morphological variation in the Mediterranean Helichrysum italicum subsp. microphyllum (Asteraceae; Gnaphalieae). Plant Biology 13: 678–687. http://dx.doi.org/10.1111/j.1438-8677.2010.00411.x, among others): (1) subsp. italicum, (2) subsp. siculum, (3) subsp. microphyllum from Crete and Cyprus, (4) subsp. microphyllum from Corsica and Sardinia, (5) subsp. microphyllum from coastal localities of Majorca and Dragonera and (6) subsp. microphyllum from Serra de Tramuntana in Majorca.
| Table 2. Number of specimens studied for each taxon (N) and their location. Previous taxonomic treatment and the final taxonomic treatment are given, the latter and the total number of individuals included in bold. |
| Country |
Geographic region |
N |
|
Taxa without taxonomic changes
|
Helichrysum italicum subsp. siculum /
Helichrysum italicum subsp. siculum
|
6
|
|
Italy
|
Sicily
|
6
|
|
Taxa with taxonomic changes
|
Helichrysum italicum subsp. italicum /
Helichrysum italicum subsp. italicum
|
33
|
|
Croatia |
1
|
|
France
|
Corsica
|
12
|
|
Greece
|
Ikaria
|
1
|
|
Naxos
|
2
|
|
Syros
|
1
|
|
Italy
|
Italian Peninsula
|
10
|
|
Elba
|
1
|
|
Pianosa
|
1
|
|
Montenegro |
2
|
|
Morocco
|
2
|
Helichrysum italicum subsp. microphyllum /
Helichrysum italicum subsp. italicum
|
5
|
|
Cyprus |
5
|
Helichrysum italicum subsp. microphyllum /
Helichrysum italicum subsp. microphyllum
|
14
|
|
Greece
|
Crete
|
14
|
Helichrysum italicum subsp. microphyllum /
Helichrysum italicum subsp. tyrrhenicum
|
46
|
|
France
|
Corsica
|
11
|
|
Italy
|
Sardinia
|
15
|
|
Spain
|
Majorca
|
15
|
| |
Dragonera
|
5
|
Helichrysum italicum subsp. microphyllum /
Helichrysum massanellanum
|
15
|
|
Spain
|
Majorca
|
15
|
|
Owing to the results of the PCA, and integrating the information on the genetic variation of the group as well (Galbany-Casals et al., 2011Galbany-Casals, M., Blanco-Moreno, J. M., Garcia-Jacas, N., Breitwieser, I. & Smissen, R. D. 2011. Genetic and morphological variation in the Mediterranean Helichrysum italicum subsp. microphyllum (Asteraceae; Gnaphalieae). Plant Biology 13: 678–687. http://dx.doi.org/10.1111/j.1438-8677.2010.00411.x), further analyses were performed, this time considering the following five groups: (1) subsp. italicum, including the specimens from Cyprus, (2) subsp. siculum, (3) subsp. microphyllum from Crete, (4) subsp. microphyllum from Corsica, Sardinia and coastal localities of Majorca and Dragonera and (5) subsp. microphyllum from Serra de Tramuntana in Majorca. Considering this grouping of provenances, we performed a Canonical Discriminant Analysis (CDA) with SPSS based on the same 30 characters to assess the morphological differentiation among the five groups described above.
Finally, significant differences in means of the morphological traits studied among the five groups defined here were tested to determine which characters could be useful for distinguishing the taxa involved. This time, three additional characters were included—hermaphroditic florets length, pistillate florets length and pappus setae length—given that they had been considered diagnostic to separate subsp. tyrrhenicum from subsp. microphyllum in a previous work (Angiolini et al., 2005Angiolini, C., Bacchetta, G., Brullo, S., Casti, M., Giusso del Galdo, G. & Guarino, R. 2005. The vegetation of mining dumps in SW-Sardinia. Feddes Repertorium 116: 243–276. http://dx.doi.org/10.1002/fedr.200411072), although these authors considered H. microphyllum at species level. First, each morphological character was evaluated to verify the normality requirements. Characters with a normal distribution were tested with one-way ANOVA analysis in conjunction with Tukey’s post-hoc multiple comparisons with SPSS. The characters that did not meet the assumptions were log-transformed. When the log-transformed variables were normally distributed, the ANOVA analysis was performed as above. For the characters for which that transformation did not improve the distribution, pairwise Kruskal-Wallis tests were performed using Bonferroni correction for multiple comparisons. This non-parametric test was performed with SPSS. All comparisons of means were performed using the mean value for each character and specimen.
RESULTSTop
In the first PCA we examined morphological variation within H. italicum. In this analysis, the first axis accounted for 19.4% of the variation and the second axis accounted for 11.9%. For the first axis, the five characters with a highest contribution to the variation and thus contributing to the differentiation of groups were: caulinar leaf length, synflorescence width, number of capitula per synflorescence, leaf margin undulation and the ratio caulinar leaf length / caulinar leaf width. For the second axis, they were: number of hermaphroditic florets per capitulum, capitula width, innermost involucral bract length, total number of florets per capitulum and capitula length.
In a first step we examined morphological variation within taxa classified as subsp. italicum, subsp. siculum and subsp. microphyllum. The graphical representation (Fig. 1) showed a transition from subsp. microphyllum to subsp. italicum specimens along the first axis, with subsp. siculum as intermediate between them. Among these groups, subsp. microphyllum showed the broadest variation in the 2-dimensional space, which suggests that different morphological entities could exist within subsp. microphyllum.
|
Figure 1. Scatterplot of the first two axes from the Principal Component Analysis (PCA) for the 119 individuals studied: 75 individuals of Helichrysum italicum subsp. microphyllum, 38 individuals of Helichrysum italicum subsp. italicum and six specimens of Helichrysum italicum subsp. siculum.
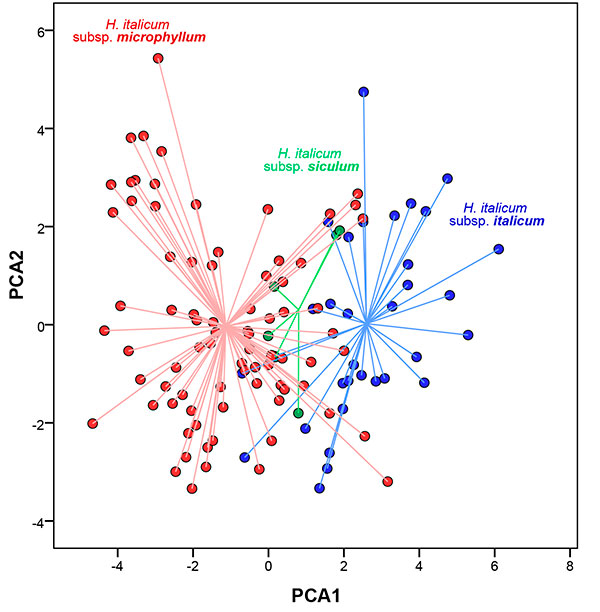
[View full size] [Descargar tamaño completo] |
|
In Fig. 2, where six groups were represented, the specimens of subsp. microphyllum from Serra de Tramuntana in Majorca were revealed as the most differentiated group, which was isolated from the rest. The following group that differed moderately from the rest was composed by the specimens of subsp. microphyllum from Crete, which represent one extreme of a morphological continuum along the first axis of variation, although they remain well differentiated from the Serra de Tramuntana group on the second axis. The remaining individuals of subsp. microphyllum from Corsica, Sardinia and coastal localities of the Balearic Islands overlapped much more, showing a notable overlap with subsp. siculum and subsp. italicum. Specimens from Cyprus (marked with black circles in Fig. 2) were included within the variation of subsp. italicum and subsp. microphyllum from coastal localities of the Balearic Islands.
|
Figure 2. Scatterplot of the first two axes from the Principal Component Analysis (PCA) for the 119 individuals studied, classified in six groups: Helichrysum italicum subsp. italicum, Helichrysum italicum subsp. siculum, Helichrysum italicum subsp. microphyllum from Crete and Cyprus (Cre, Cyp), Helichrysum italicum subsp. microphyllum from Corsica and Sardinia (Cor, Sar), Helichrysum italicum subsp. microphyllum from coastal localities of Majorca and Dragonera (Mll c, Dra), and Helichrysum italicum subsp. microphyllum from Serra de Tramuntana in Majorca (Mll m). Circles with thicker black border correspond to specimens from Cyprus.
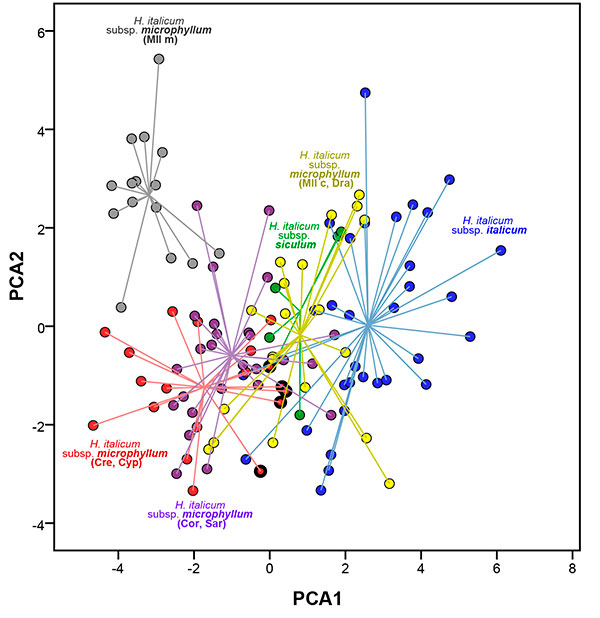
[View full size] [Descargar tamaño completo] |
|
In the CDA the first two components explained 80.8% of total variance (CDA1 = 58.0%; CDA2 = 22.8%). The individuals were distributed in two main clouds (Fig. 3): one composed by subsp. microphyllum from Serra de Tramuntana (named Helichrysum massanellanum from now on), which was clearly isolated from another main cloud, which grouped H. italicum subsp. italicum, H. italicum subsp. microphyllum from Crete and H. italicum subsp. microphyllum from Corsica, Sardinia and coastal localities in Majorca and Dragonera (the last group considered as subsp. tyrrhenicum from now on) and somewhat farther apart H. italicum subsp. siculum. Within this second cloud, the four predefined groups were shown as rather clearly separated from each other, with only some overlapping among them. The characters that were most correlated with the first canonical axis and that contributed to the separation of H. massanellanum from the rest of the groups were leaf margin undulation, caulinar leaf length, caulinar leaf length / caulinar leaf width, synflorescence width and number of capitula per synflorescence. Those correlating with the second axis, which contributed to the differentiation of the rest of the groups, were eglandular indumentum of leaf adaxial side, average of innermost involucral bract length / outermost involucral bract length, caulinar leaf length / caulinar leaf width, caulinar leaf width and innermost involucral bract length (Appendix 2). Total percentage of correctly classified individuals in the predefined groups was 95.8%. Helichrysym massanellanum, H. italicum subsp. siculum and H. italicum subsp. microphyllum resulted in 100% correct classification and H. italicum subsp. italicum and H. italicum subsp. tyrrhenicum revealed high values of correctly classified plants with 94.7% and 93.5%, respectively (Appendix 3). Specimens from Cyprus, considered in this analysis under subsp. italicum, were clearly included under the morphological variation of this subspecies.
|
Figure 3. Scatterplot of canonical scores on the first two axes resulting from applying the discriminant functions considering the 30 morphometric variables for the 119 individuals studied classified in five predefined groups. Circles with thicker black border correspond to specimens from Cyprus.
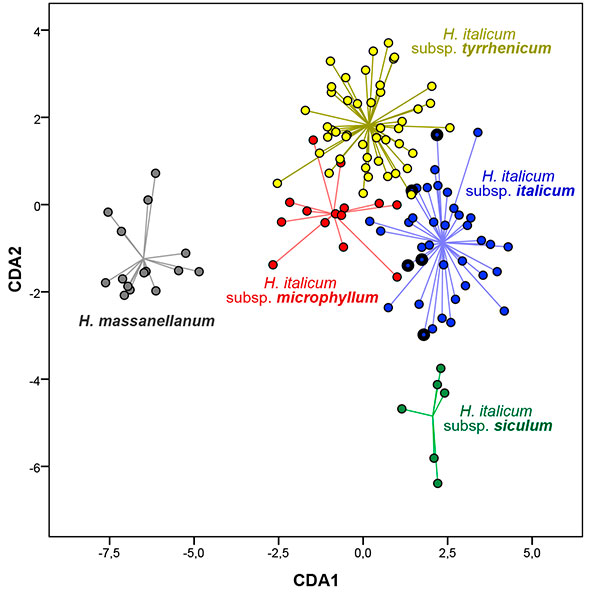
[View full size] [Descargar tamaño completo] |
|
Finally, ANOVA confirmed strong morphological differentiation of H. massanellanum from H. italicum subspecies (Appendix 4), in congruence with the PCA (Fig. 2) and the CDA (Fig. 3). The most relevant results were that H. massanellanum differed significantly from the other four groups in having less axillary leaf fascicles and longer outermost involucral bracts, and additionally differed from all taxa except for subsp. siculum in having longer and wider capitula, and a higher number of hermaphroditic florets per capitulum. Helichrysum italicum subsp. italicum differed from the rest in having wider synflorescences with a higher number of capitula, from the other subspecies in having fewer axillary leaf fascicles, and differed from subsp. tyrrhenicum and subsp. microphyllum in having longer leaves. Helichrysum italicum subsp. microphyllum differed from the other subspecies in having leaf margins more markedly undulate. Helichrysum italicum subsp. siculum differed from the rest in having longer innermost bracts in relation to the outermost and from other subspecies of H. italicum in having a lower density of eglandular hairs on the adaxial side of leaves, whereas subsp. tyrrhenicum differed from the rest in having a higher density of eglandular hairs on the adaxial side of leaves.
Focusing on the new subdivision established within the variation of the old widely circumscribed subsp. microphyllum, it is worth highlighting that the newly cirscumscribed subsp. microphyllum as considered only from Crete differed from subsp. tyrrhenicum—from Corsica, Sardinia and coastal localities of the Balearic Islands—in having more markedly undulate leaves, a lower density of eglandular hairs on the adaxial side of leaves and a lower number of pistillate florets.
DISCUSSIONTop
Detailed morphometric analyses used here shed light on delimitation of morphological entities within Helichrysum italicum and clarify their taxonomic status. Plants from Majorca mountain area (Serra de Tramuntana), traditionally identified as H. italicum subsp. microphyllum, should be considered as a separate taxon due to the well-defined morphological traits providing further arguments for elevating them to species level. Several authors (Pla et al., 1992Pla, V., Sastre, B. & Llorens, L. 1992. Aproximació al catàleg de la flora vascular de les illes Balears: 1992. Universitat de les Illes Balears, Palma.; Sáez & Rosselló, 2001Sáez, L. & Rosselló, J. A. 2001. Llibre Vermell de la flora amenaçada de les IIles Balears. Conselleria de Medi Ambient (Govern de les Illes Balears), Palma de Mallorca.; Galbany-Casals et al., 2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082) had previously suggested the existence of some morphological differences between mountain and coastal populations from Majorca, but without giving any diagnosis or publishing the description of a new taxon. In this study, we have shown that the degree of morphological differentiation is much higher between H. massanellanum and any of the other taxa of the group than between any pair among the rest of the taxa (Fig. 3). Its morphological distinctness is noticeable in the context of the overall morphological variation of the group and makes the species level more appropriate for these populations. The taxonomically most significant and particular traits to consider H. massanellanum as a distinct species include appressed caulinar leaves instead of these being erecto-patent to patent, absence of axillary leaf fascicles—they are only very rarely present—and longer innermost involucral bracts than other taxa. Additionally, it differs from all taxa except for subsp. siculum in having longer and wider capitula, more hermaphroditic florets per capitulum and, except for subsp. microphyllum, in having shorter caulinar leaves. Plants from Serra de Tramuntana had been traditionally considered more similar and possibly closely related to populations from Crete (Jeanmonod, 1998Jeanmonod, D. 1998. Xanthium subg. Xanthium et Helichrysum italicum deux cas taxonomiques ardus. Candollea 53: 435–457.; Galbany-Casals et al., 2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082). However, the similarity in general appearance between populations from Serra de Tramuntana and Crete would be attributed to parallel evolution in adaptation to mountain open shrubby formations (Figs. 2, 4A and 4B), given that published results based on AFLP and DNA sequences demonstrated that both sets of populations are not genetically closely related (Galbany-Casals et al., 2011Galbany-Casals, M., Blanco-Moreno, J. M., Garcia-Jacas, N., Breitwieser, I. & Smissen, R. D. 2011. Genetic and morphological variation in the Mediterranean Helichrysum italicum subsp. microphyllum (Asteraceae; Gnaphalieae). Plant Biology 13: 678–687. http://dx.doi.org/10.1111/j.1438-8677.2010.00411.x). Moreover, Helichrysum massanellanum is here shown to be morphologically well differentiated from the geographically close H. italicum subsp. tyrrhenicum, which is found in Majorca as well, but is restricted to coastal localities and Dragonera islet (Figs. 3, 4A and 4D). Helichrysum massanellanum is restricted to a small area in Massanella massif (Serra de Tramuntana, Majorca, Balearic Islands), between (700)900 and 1360 m a.s.l. This is an area with an extraordinary concentration of narrow endemic plant species, such as Arenaria bolosii (Cañig.) L. Sáez & Rosselló, Chaenorhinum rodriguezii (Porta) L. Sáez & Vicens and Euphorbia fontqueriana Greuter.
|
Figure 4. General aspect of Helichrysum massanellanum and the newly accepted subspecies of Helichrysum italicum traditionally considered within subsp. microphyllum. (A), Helichrysum massanellanum, Majorca, Galbany & Sáez s. n. (BCN 20580); (B), Helichrysum italicum subsp. microphyllum, Crete, Galbany 2006 et al. (BC 948656); (C), Helichrysum italicum subsp. tyrrhenicum, Corsica, Galbany 2052 & Arrabal (BC 949617); (D), Helichrysum italicum subsp. tyrrhenicum, Majorca, Mus s. n. (Herbari Universitat Illes Balears). (A), Centre de Documentació de Biodiversitat Vegetal de la Universitat de Barcelona. Reproduction authorized; (B) and (C), © Institut Botànic de Barcelona CSIC-Ajuntament de Barcelona. Reproduction authorized; (D) M. Mus. Reproduction authorized.
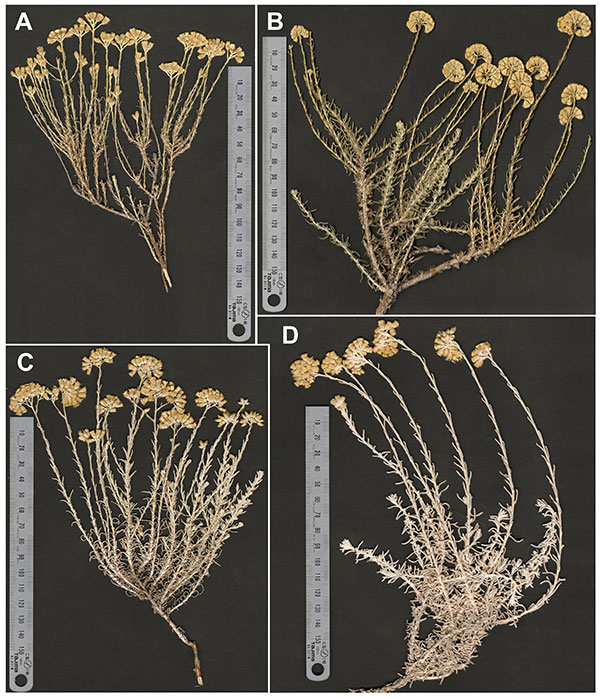
[View full size] [Descargar tamaño completo] |
|
With regards to H. italicum, as a result of considering morphological features, genetic groups and geographical distribution, the final taxonomic treatment adopted here recognizes four taxa at subspecies-level: Helichrysum italicum subsp. italicum (Figs. 5C and 5D), H. italicum subsp. siculum (Fig. 5B), H. italicum subsp. microphyllum (Fig. 4B) and H. italicum subsp. tyrrhenicum (Figs. 4C, 4D and 5A). It is necessary to highlight that gene flow is notable within the whole group and that both genetic and morphological differences are not clear-cut but present a geographic gradient. Therefore, separation among taxa is not easy in areas where two taxa overlap or grow in close proximity, such as Corsica, where subsp. italicum and subsp. tyrrhenicum coexist, or the eastern Mediterranean area, where subsp. italicum morphologically approaches subsp. microphyllum. The documented gene flow and the morphological overlapping between these populations suggests that subspecies level is the most appropriate taxonomic rank for these taxa, which together constitute a well differentiated group from H. massanellanum (Fig. 3). At the same time, H. italicum harbors a noticeable morphological variation, with subsp. italicum the most variable and widely distributed subspecies, and each of the other subspecies rather well defined by a combination of morphological traits and a well-delimited geographic area. A map comparing past and new taxonomic classification and the distribution areas of each taxa is shown in Fig. 6. All of them have undergone a taxonomical and geographical recircumscription, except for subsp. siculum, which is considered as in Galbany-Casals et al. (2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082), endemic to Sicily, and which presents some genetic distinctness in the context of the group (Galbany-Casals et al., 2011Galbany-Casals, M., Blanco-Moreno, J. M., Garcia-Jacas, N., Breitwieser, I. & Smissen, R. D. 2011. Genetic and morphological variation in the Mediterranean Helichrysum italicum subsp. microphyllum (Asteraceae; Gnaphalieae). Plant Biology 13: 678–687. http://dx.doi.org/10.1111/j.1438-8677.2010.00411.x).
|
Figure 5. General aspect of some subspecies of Helichrysum italicum. (A), Helichrysum italicum subsp. tyrrhenicum, Sardinia, Nieto & Fuertes s. n. (BCN 20711); (B), Helichrysum italicum subsp. siculum, Sicily, Galbany s. n. (BCN 25234); (C), Helichrysum italicum subsp. italicum, Cyprus, Galbany 2038 et al. (BC 948593); (D), Helichrysum italicum subsp. italicum, Naxos, Galbany 2003 et al. (BC 948657). (A) and (B), Centre de Documentació de Biodiversitat Vegetal de la Universitat de Barcelona. Reproduction authorized; (C) and (D), © Institut Botànic de Barcelona CSIC-Ajuntament de Barcelona. Reproduction authorized.

[View full size] [Descargar tamaño completo] |
|
|
Figure 6. Distribution map of studied taxa, modified from Galbany-Casals et al. (2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082). (A), taxonomic treatment presented in Galbany-Casals et al. (2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082); (B), new taxonomic treatment proposed here. The box in the upper left corner shows Majorca in more detail.
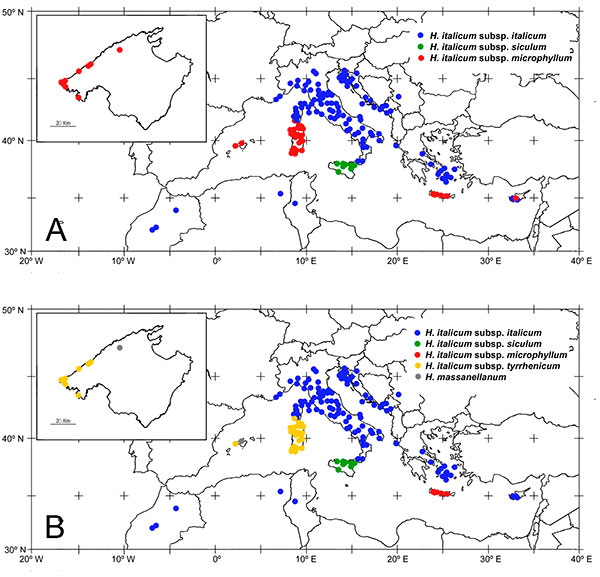
[View full size] [Descargar tamaño completo] |
|
Helichrysum italicum subsp. microphyllum, in a traditional sense, displays geographically structured morphological variation and this is also reflected in genetic structure (Galbany-Casals et al., 2011Galbany-Casals, M., Blanco-Moreno, J. M., Garcia-Jacas, N., Breitwieser, I. & Smissen, R. D. 2011. Genetic and morphological variation in the Mediterranean Helichrysum italicum subsp. microphyllum (Asteraceae; Gnaphalieae). Plant Biology 13: 678–687. http://dx.doi.org/10.1111/j.1438-8677.2010.00411.x). The CDA analysis provides statistical support for the morphological discrimination of the populations distributed in western Mediterranean Basin (Corsica, Sardinia, Majorca coastline and Dragonera) from those from Crete, corresponding to the western and eastern groups detected by Galbany-Casals et al. (2011Galbany-Casals, M., Blanco-Moreno, J. M., Garcia-Jacas, N., Breitwieser, I. & Smissen, R. D. 2011. Genetic and morphological variation in the Mediterranean Helichrysum italicum subsp. microphyllum (Asteraceae; Gnaphalieae). Plant Biology 13: 678–687. http://dx.doi.org/10.1111/j.1438-8677.2010.00411.x) according to genetic data. It is interesting to note that the morphological divergence between populations from these two regions is probably due to local, independent selection acting on the same gene pool of H. italicum (Galbany-Casals et al., 2011Galbany-Casals, M., Blanco-Moreno, J. M., Garcia-Jacas, N., Breitwieser, I. & Smissen, R. D. 2011. Genetic and morphological variation in the Mediterranean Helichrysum italicum subsp. microphyllum (Asteraceae; Gnaphalieae). Plant Biology 13: 678–687. http://dx.doi.org/10.1111/j.1438-8677.2010.00411.x). These evidences lead us to lend support to the recognition of two separate taxa (subsp. microphyllum and subsp. tyrrhenicum), both being probably originated independently from a common gene pool with subsp. italicum (Galbany-Casals et al., 2011Galbany-Casals, M., Blanco-Moreno, J. M., Garcia-Jacas, N., Breitwieser, I. & Smissen, R. D. 2011. Genetic and morphological variation in the Mediterranean Helichrysum italicum subsp. microphyllum (Asteraceae; Gnaphalieae). Plant Biology 13: 678–687. http://dx.doi.org/10.1111/j.1438-8677.2010.00411.x). Hence, following the nomenclatural priority, the name H. italicum subsp. microphyllum should be applied to plants from Crete, from where it was originally described (Willdenow, 1803Willdenow, C. L. 1803. Caroli a Linné Species Plantarum [...] editio quarta [...], 3(3). Impensis G. C. Nauk, Berolini.; as Gnaphalium microphyllum Willd.). Helichrysum italicum subsp. tyrrhenicum was originally described in a previous work (Angiolini et al., 2005Angiolini, C., Bacchetta, G., Brullo, S., Casti, M., Giusso del Galdo, G. & Guarino, R. 2005. The vegetation of mining dumps in SW-Sardinia. Feddes Repertorium 116: 243–276. http://dx.doi.org/10.1002/fedr.200411072) as Helichrysum microphyllum (Willd.) Cambess. subsp. tyrrhenicum Bacch., Brullo & Giusso. These authors observed morphological differences between Cretan specimens (H. microphyllum subsp. microphyllum) and plants from western Mediterranean Basin (Corsica, Sardinia, Balearic Islands) in innermost involucral bracts length, florets length and pappus setae length (Angiolini et al., 2005Angiolini, C., Bacchetta, G., Brullo, S., Casti, M., Giusso del Galdo, G. & Guarino, R. 2005. The vegetation of mining dumps in SW-Sardinia. Feddes Repertorium 116: 243–276. http://dx.doi.org/10.1002/fedr.200411072). We reevaluated the same characters and did not find significant differences in their mean values between these two taxa (Appendix 4). However, we found that subsp. microphyllum (including only the Cretan plants) differs from subsp. tyrrhenicum—which is considered in the present work to be distributed in Corsica, Sardinia and coastal localities of the Balearic Islands—in having more markedly undulate leaves, a lower density of eglandular hairs in the leaf adaxial side and a lower number of pistillate florets (Appendix 4).
As regards to the classification of plants from Cyprus, a recent taxonomic treatment (Galbany-Casals et al., 2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082) considered these populations within a broad concept of H. italicum subsp. microphyllum, mainly due to similar morphological characters of these populations and specimens from Corsica and Sardinia, such as the densely tomentose and relatively short leaves and the abundant presence of axillary leaf fascicles. However, considering the new taxonomic scenario presented here where subsp. microphyllum is fragmented in three different taxa—H. massanellanum, H. italicum subsp. tyrrhenicum and H. italicum subsp. microphyllum—, the identity of the Cypriot plants must be reevaluated.
According to the three genetic groups found within H. italicum s. l. in the Mediterranean Basin (Galbany-Casals et al., 2011Galbany-Casals, M., Blanco-Moreno, J. M., Garcia-Jacas, N., Breitwieser, I. & Smissen, R. D. 2011. Genetic and morphological variation in the Mediterranean Helichrysum italicum subsp. microphyllum (Asteraceae; Gnaphalieae). Plant Biology 13: 678–687. http://dx.doi.org/10.1111/j.1438-8677.2010.00411.x), populations from Cyprus would belong to the eastern genetic group. However, if we compare the morphological characters of subsp. microphyllum from Crete and individuals from Cyprus, no taxonomically relevant characters are shared. Actually, Cypriot individuals appear to be morphologically intermediate between subsp. italicum and Cretan specimens but within subsp. italicum variation range. Considering together morphological similarities and genetic structure of the whole group, we suggest that specimens from Cyprus are better included in subsp. italicum. This is consistent with the work of previous authors who considered the Cyprus populations to belong to subsp. italicum (Boissier, 1840Boissier, E. 1840. Voyage botanique dans le midi de l’Espagne pendant l’année 1837 2. Gide et Cie, Paris.; Holmboe, 1914Holmboe, J. 1914. Studies on the vegetation of Cyprus: Based upon researches during the spring and summer 1905. John Griegs, Bergen.; Georgiadou, 1985Georgiadou, E. 1985. Helichrysum. In: Meikle, R. D. (Ed.), Flora of Cyprus 2. Royal Botanic Gardens, Kew: 888–889.; Greuter, 2008Greuter, W. 2008. Helichrysum Mill. In: Greuter, W. & Raab-Straube, E. von (Eds.), Med-checklist 2 Dicotyledones (Compositae). Organisation for the Phyto-Taxonomic Investigation of the Mediterranean Area (OPTIMA), Genève: 234–239.), and is reinforced by our numerical results from the CDA analysis (Fig. 3), where Cypriot specimens were well classified in the subsp. italicum group.
The present study has shown the morphological differentiation of H. massanellanum from H. italicum, probably influenced by geographic isolation and ecological adaptation. Geographic, genetic and morphological gradient found in H. italicum in the whole Mediterranean Basin explains our new taxonomic treatment of H. italicum splitting it in four taxa at subspecies level.
TAXONOMIC TREATMENTTop
Based on the discussion above, the following taxonomic treatment with a standard identificaction key is presented. Due to morphological overlapping, several specimens should be examined to aid in the correct species identification. Additionally, we also provide a morphological description based on our own measurements, habitat requirements, distribution range and phenology for each taxon. A complete nomenclatural treatment is presented for all taxa, although types are only cited for the accepted taxa. Type information for the other published names is compiled in Galbany-Casals et al. (2006bGalbany-Casals, M., Sáez, L. & Benedí, C. 2006b. Conspectus of Helichrysum Mill. Sect. Stoechadina (DC.) Gren & Godr. (Asteraceae, Gnaphalieae). Orsis 21: 58–81. , cGalbany-Casals, M., Sáez, L., Benedí, C. & Jarvis, C. E. 2006c. Typification of names in Gnaphalium L. and Helichrysum Mill. (Asteraceae), and some taxonomic notes. Taxon 55: 489–501. http://dx.doi.org/10.2307/25065597).
Key to Helichrysum massanellanum and Helichrysum italicum
1. Plant without axillary leaf fascicles—exceptionally very few of them—in flowering and vegetative stems. Basal and medial leaves of the flowering stems appressed
........................................................................................................1. H. massanellanum
-. Plant with some or abundant axillary leaf fascicles—exceptionally without them—in flowering and vegetative stems. Basal and medial leaves of the flowering stems erecto-patent to patent
........................................................................................................2. H. italicum
1. Helichrysum massanellanum Herrando, J. M. Blanco, L. Sáez & Galbany, sp. nov.
Type: [Spain. Balearic Islands] Mallorca: Serra de Tramuntana, Coll de Ses Cases de Neu, c. Coll des Telègraf, 1205 m, Ca., 21.06.2001, M. Galbany & L. Sáez s. n. (holotype: BCN 20580! (Fig. 7); isotype: BC 939749!).
Diagnosis: Helichrysum massanellanum is morphologically similar to H. italicum, with which it shares cylindrical to cylindrical-campanulate capitula and outermost involucral bracts linear-lanceolate to lanceolate, partially or completely herbaceous and covered with a dense indumentum. If differs, however, from this species in that it has appressed basal and medial caulinar leaves and it does not have axillary leaf fascicles (only rarely few of them), whereas H. italicum has erecto-patent to patent basal and medial caulinar leaves and it generally has axillary leaf fascicles at least in some leaves. Additionally, Helichrysum massanellanum differs from H. italicum in having, in general, shorter caulinar leaves, longer and wider capitula, and more hermaphroditic florets per capitulum.
Subshrubby perennial, aromatic, up to 40 cm high (Fig. 4A). Herbaceous parts of vegetative and flowering stems 3.2–14.4 cm, densely tomentose, erect, leafy all their length, generally without axillary leaf fascicles—exceptionally very few of them. Basal and medial leaves of the flowering stems appresed, more densely distributed in the basal part of the stem, diminishing size towards the synflorescence and becoming laxer. Basal and medial leaves on vegetative and flowering stems 2–7(10) × 0.5–1(1.3) mm, linear-lanceolate, margin revolute and undulate, with obtuse to rounded tips, sessile and subdecurrent at the base, markedly discolorous, subglabrous or arachnoid, rarely arachnoid-tomentose, and sparsely glandular on the adaxial surface, densely tomentose and sparsely to densely glandular on the abaxial surface. Synflorescence corymbose, terminal, (4.5)6–14(32) × (3)6–16(21) mm, with 1–10(18) pedunculate capitula. Capitula disciform, heterogamous, very rarely discoid, homogamous, (4)4.8–7 × 2.5–4(5) mm, cylindrical to narrowly campanulate (Fig. 8A); involucral bracts 19–38 per capitulum, densely imbricate, approximately as long as the florets, papery, yellow, except for the outermost ones which are completely herbaceous, rarely only in their proximal half (Fig. 8B); outermost bracts (1.2)1.5–2.5 × 0.5–1.2 mm, linear-lanceolate to lanceolate, with acute to subobtuse tips, arachnoid-tomentose to tomentose, often also arachnoid in its inner face, sparsely to densely glandular; middle bracts ovate, obovate or elliptic; innermost bracts (3.5)4–5.4 × 0.5–1.4 mm, linear, narrowly lanceolate, oblanceolate or narrowly spatulate, with acute, obtuse or rounded tips, glabrous to arachnoid and sparsely to densely glandular in the stereome. Receptacle flat, alveolate. Florets (15)18–30 per capitulum; pistillate (0)3–10 per capitulum, corolla 2.4–4 mm, narrowly tubular; hermaphroditic (11)16–26 per capitulum, corolla (2.4)3–4.3 mm, tubular and narrowly campanulate above; corolla yellow. Achenes 0.8–1 × 0.3–0.6 mm, cylindrical to ovoid, with regularly scattered white duplex hairs, sometimes mixed with amber multicellular biseriate glandular hairs. Pappus 2.6–4.2 mm, white, uniseriate, constituted by free scabrid setae with apical cells acute or obtuse and patent cilia at the base.
|
Figure 8. Capitula and involucral bract series, from the outermost (left) to the innermost (right). (A) and (B), Helichrysum massanellanum, Majorca, Galbany & Sáez s. n. (BCN 20580); (C) and (D), Helichrysum italicum subsp. microphyllum, Crete, Galbany 2006 et al. (BC 948656); (E) and (F), Helichrysum italicum subsp. tyrrhenicum, Sardinia, Galbany & Sáez s. n. (BCN 25235); (G) and (H), Helichrysum italicum subsp. italicum, Naxos, Galbany 2003 et al. (BC 948656); (I) and (J), Helichrysum italicum subsp. siculum, Sicily, Galbany s. n. (BCN 24031). Scale bar = 1 mm. Drawings by M. Galbany-Casals and L. Sáez.
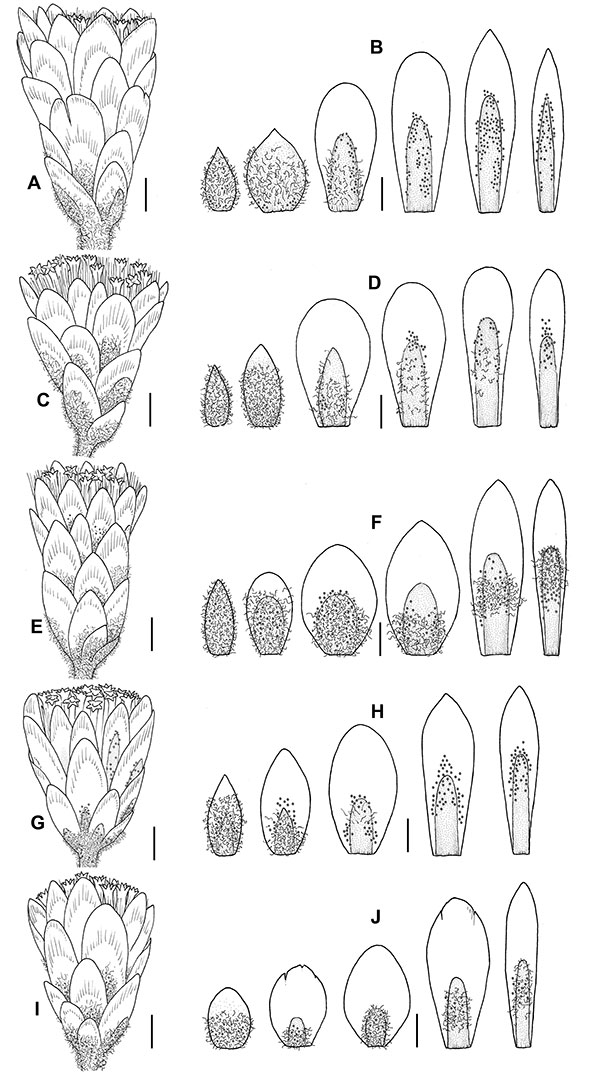
[View full size] [Descargar tamaño completo] |
|
Etymology: The specific epithet refers to the Majorcan Massanella massif, in Serra de Tramuntana, on which this species occurs.
Distribution and habitat: Helichrysum massanellanum (Fig. 9) is restricted to a small area in Massanella massif (Serra de Tramuntana, Majorca, Balearic Islands) (Fig. 6). This is an area with an extraordinary concentration of endemic plant species, most of them with restricted patterns of regional occurrence (Sáez, 2010Sáez, L. 2010. Plantes endèmiques dels Països Catalans. In: Giralt, J. (Ed.), Història natural dels Països Catalans, Suplement fauna i flora. Enciclopèdia Catalana, Barcelona: 161–164. ; López-Pujol et al.López-Pujol, J., Martinell, M. C., Massó, S., Blanché, C. & Sáez, L. 2013. The ‘paradigm of extremes’: extremely low genetic diversity in an extremely narrow endemic species, Coristospermum huteri (Umbelliferae). Plant Systematics and Evolution 299: 273–275. http://dx.doi.org/10.1007/s00606-012-0732-3, 2013). It grows between (700)900 and 1360 m a.s.l., mainly on calcareous stony slopes facing north. It occupies open places, in which Ampelodesmos mauritanicus (Poir.) Durand & Schinz is common, with scarce woody plants cover. Helichrysum massanellanum grows together with several endemic shrub species such as Genista valdes-bermejoi Talavera & L. Sáez, Hypericum balearicum L., Santolina magonica (O. Bolòs, Molinier & P. Monts.) Romo and Thymelaea velutina (Cambess.) Endl., and some herbaceous endemic species such as Arenaria bolosii (Cañig.) L. Sáez & Rosselló, Chaenorhinum rodriguezii (Porta) L. Sáez & Vicens and Euphorbia fontqueriana Greuter. The area of occupancy is ca. 2 km2 and the extent of occurrence is ca. 15 km2.
Phenology: Flowering specimens of H. massanellanum can be found from late May to August.
Conservation status: According to the IUCN (2001IUCN (International Union for Conservation of Nature) 2001. IUCN Red List categories and criteria Version 3.1. IUCN Species Survival Commission, Gland,& Cambridge.), H. massanellanum should be included in the “Vulnerable” category (VU D2).
2. Helichrysum italicum (Roth) G. Don in Loudon, Hort. Brit. 342 (1830).
≡ Gnaphalium italicum Roth in Bot. Mag. (Römer & Usteri) 4 (10): 19 (1790). ≡ Helichrysum italicum (Roth) Guss., Fl. Sicul. Syn. 2: 469 (1844), comb. superfl. ≡ H. angustifolium subsp. italicum (Roth) Briq. & Cavill. in Burnat, Fl. Alpes Marit. 6 (2): 265 (1917).
Neotype (designated by Georgiadou (1985Georgiadou, E. 1985. Helichrysum. In: Meikle, R. D. (Ed.), Flora of Cyprus 2. Royal Botanic Gardens, Kew: 888–889.: 888): “Gnaphalium italicum” (B-W No. 15445!).
Subshrubby perennial, aromatic, up to 30–70 cm high. Herbaceous parts of vegetative and flowering stems 3.2–36.1(40.5) cm, densely tomentose, erect to ascendent-erect, leafy all their length, with some or abundant axillary leaf fascicles—exceptionally without them. Basal and medial leaves of the flowering stems erecto-patent to patent, more densely distributed in the basal part of the stem, diminishing in size towards the synflorescence and becoming laxer and apressed to the stem. Basal and medial leaves on vegetative and flowering stems 3–37 × 0.4–1.7(1.8) mm, linear to linear-lanceolate, margin revolute and undulate or not, with obtuse to rounded tips, sessile and subdecurrent at the base, concolorous or discolorous, subglabrous, arachnoid or tomentose and eglandular to sparsely glandular on the adaxial surface, tomentose to densely tomentose and eglandular to densely glandular on the abaxial surface. Axillary leaves, when present, 1.5–9.5(11) × 0.4–1.5 mm. Synflorescence corymbose, terminal, 6–47(69) × 8.5–55(80) mm, with 3–95(120) pedunculate capitula. Capitula disciform, heterogamous, (3.5)4–6.5 × 2–4(4.6) mm, cylindrical to narrowly campanulate; involucral bracts 18–40 per capitulum, densely imbricate, approximately as long as the florets, papery, yellow, except for the outermost ones which are herbaceous at least in their proximal half; outermost bracts (0.7)1–2.5 × 0.4–1.8 mm, often linear-lanceolate to lanceolate, sometimes ovate-lanceolate, with acute to obtuse tips, arachnoid-tomentose to tomentose, rarely arachnoid, eglandular to densely glandular; middle bracts ovate, obovate or elliptic; innermost bracts (2.5)3–5.5 × 0.3–1.4 mm, linear, narrowly lanceolate or narrowly spatulate, with acute, obtuse or rounded tips, glabrous to arachnoid-tomentose, sparsely to densely glandular in the stereome. Receptacle flat, alveolate. Florets (8)13–36(39) per capitulum; pistillate 1–10(12) per capitulum, corolla 2–4.1 mm, narrowly tubular; hermaphroditic (7)9–29 per capitulum, corolla (2.5)2.7–4.4 mm, tubular and narrowly campanulate above; corolla yellow. Achenes 0.6–1.1 × 0.2–0.7 mm, cylindrical to ovoid, with regularly scattered white duplex hairs, sometimes glabrous. Pappus 2.1–4.3 mm, white, uniseriate, constituted by free scabrid setae with apical cells acute or obtuse and patent cilia at the base.
Key to the subspecies of Helichrysum italicum
1. Basal and medial leaves markedly discolorous, subglabrous—rarely arachnoid—in the adaxial surface and densely tomentose in the abaxial surface
........................................................................................................ 2
-. Basal and medial leaves concolorous or almost so, arachnoid to tomentose in the adaxial surface and densely tomentose in the abaxial surface
........................................................................................................ 4
2. Plant up to 70 cm high, with some axillary leaf fascicles—exceptionally without them. Basal and medial leaves of flowering and vegetative stems (8)12–37 mm long. Synflorescence 10–35(62) × (15)18–55(80) mm, with (8)15–94(120) capitula
........................................................................................................2a. H. italicum subsp. italicum
-. Plant up to 40 cm high, with abundant axillary leaf fascicles. Basal and medial leaves of flowering and vegetative stems 3–18.5(21) mm long. Synflorescence 6–23(35) × 8.5–27(37) mm, with 3–36(42) capitula
........................................................................................................3
3. Basal and medial leaves of flowering and vegetative stems (7)10–18.5(21) mm long, with the margin not undulate
........................................................................................................2b. H. italicum subsp. siculum
-. Basal and medial leaves of flowering and vegetative stems 3–10(14.3) mm long, with the margin generally undulate
........................................................................................................2c. H. italicum subsp. microphyllum
4. Plant up to 40 cm high, with abundant axillary leaf fascicles. Basal leaves of flowering and vegetative stems 3.7–20(29) mm long. Synflorescence (9.3)14–37(51.5) mm wide, with 5–37(64) capitula
........................................................................................................2d. H. italicum subsp. tyrrhenicum
-. Plant up to 70 cm high, with some axillary leaf fascicles—exceptionally without them. Basal leaves of flowering and vegetative stems (8)12-37 mm long. Synflorescence (15)18–55(80) mm wide, with (8)15–94(120) capitula
........................................................................................................2a. H. italicum subsp. italicum
2a. Helichrysum italicum subsp. italicum
= Helichrysum serotinum var. orientale Boiss., Voy. Bot. Espagne 2: 328 (1840).
= Helichrysum numidicum Pomel, Nouv. Mat. Fl. Atl. 2: 288 (1875). ≡ H. angustifolium var. numidicum (Pomel) Maire in Mém. Soc. Sci. Nat. Maroc 22 (1): 37 n.º 61 (1930).
= Helichrysum angustifolium var. brevifolium Rouy, Fl. France 8: 193 (1903).
= Helichrysum angustifolium var. longifolium Rouy, Fl. France 8: 193 (1903).
= Helichrysum italicum var. ericoideum Fiori in Fiori & Paol., Fl. Italia 3: 283 (1904).
= Helichrysum italicum var. canum Holmboe, Stud. Veg. Cyprus: 179 (1914).
Plants up to 70 cm high (Figs. 5C and 5D). Herbaceous parts of vegetative and flowering stems (6.4)18.5–36.1(40.5) cm, with some axillary leaf fascicles—exceptionally without them. Basal and medial leaves on vegetative and flowering stems (8)12–37 × 0.4–1.2(1.8) mm, linear, margin revolute and not undulate, concolorous, rarely discolorous, subglabrous to arachnoid-tomentose and eglandular to sparsely glandular on the adaxial surface. Axillary leaves, when present, (3)4–9.5(11) × 0.5–1.3 mm. Synflorescence 10–35(62) × (15)18–55(80) mm, with (8)15–94(120) capitula. Capitula 4–6.5 × 2–4 mm, cylindrical to narrowly campanulate (Fig. 8G); involucral bracts 22–40 per capitulum (Fig. 8H); outermost bracts 1–2.2(3) × 0.4–1.5 mm, linear-lanceolate to lanceolate, with acute to subobtuse tips, arachnoid-tomentose to tomentose, rarely arachnoid, eglandular to densely glandular; innermost bracts 3.2–5(5.5) × (0.5)0.7–1.2 mm, linear-lanceolate, with acute, obtuse or rounded tips, glabrous to arachnoid-tomentose and sparsely to densely glandular in the stereome. Florets 14–31(34) per capitulum; pistillate (2)4–10 per capitulum, corolla (2.3)2.5–4 mm; hermaphroditic 9–22(26) per capitulum, corolla 2.7–4.4 mm. Achenes ca. 0.9–1 × 0.4–0.5 mm, cylindrical to ovoid, with regularly scattered white duplex hairs. Pappus 2.2–4 mm.
Distribution and habitat: Widespread in Italy and Croatia, it extends also to the eastern Mediterranean coast of France and Corsica, Bosnia–Herzegovina, Serbia and Montenegro, Slovenia, Greece, mainly Aegean Islands, and Cyprus, as well as in scattered, isolated localities in Algeria, Morocco and Tunisia (Fig. 6). It grows in a great diversity of open habitats, including several shrubby and herbaceous formations, road banks and path margins, maritime rocks, cliffs and sand dunes as well as on several types of substrate, granitic, schistose, volcanic, or limestone rocky soils. It is very common in its geographic area and is often one of the first pioneer plant to colonize disturbed areas. Altitudinal range: 0–2200 m.
Phenology: Flowering specimens of H. italicum subsp. italicum can be found from (May) June to August (September).
2b. Helichrysum italicum subsp. siculum (Jord. & Fourr.) Galbany, L. Sáez & Benedí in Canad. J. Bot. 84(8): 1225 (2006).
≡ H. siculum Jord. & Fourr., Brev. Pl. Nov. 2: 67 (1868) [basionym].
Lectotype (designated by Galbany-Casals et al. (2006aGalbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082: 1225): [Italy] Sicile, 1849, Gussone s.n. [ex herb. Al. Jordan 187] (lectotype: LY photo!; isolectotype: MPU!).
Plants up to 40 cm high (Fig. 5B). Herbaceous parts of vegetative and flowering stems (5.5)9.2–25.9(34.5) cm, with abundant axillary leaf fascicles. Basal and medial leaves on vegetative and flowering stems (7)10–18.5(21) × 0.6–1.1 mm, linear, margin revolute and not undulate, markedly discolorous, subglabrous, rarely arachnoid, and sparsely glandular on the adaxial surface. Axillary leaves (2)3–5.9 × 0.5–1 mm. Synflorescence (6)10–25 × 12–26 mm, with 3–26(42) capitula. Capitula 4–6 × 2.2–4 mm, cylindrical to narrowly campanulate (Fig. 8I); involucral bracts 26–36 per capitulum (Fig. 8J); outermost bracts 1–1.5(1.9) × 0.4–1.4 mm, ovate-lanceolate, with obtuse tips, arachnoid-tomentose to tomentose, sparsely to densely glandular; innermost bracts 3–5 × 0.8–1.4 mm, linear, narrowly lanceolate, with acute, obtuse or rounded tips, arachnoid to arachnoid-tomentose and sparsely to densely glandular in the stereome. Florets 15–27 per capitulum; pistillate 3–8 per capitulum, corolla 2.5–3.8(4.1) mm; hermaphroditic 11–20 per capitulum, corolla 3–4.4 mm. Achenes ca. 0.8–0.9 × 0.3–0.5 mm, cylindrical to ovoid, with regularly scattered white duplex hairs. Pappus 2.5–4.3 mm.
Distribution and habitat: Helichrysum italicum subsp. siculum is endemic to Sicily (Fig. 6). This subspecies grows in a great diversity of open habitats, including several shrubby and herbaceous formations, road banks and path margins, beds of temporary streams, maritime rocks and sand dunes; as well as on several types of substrates, such as volcanic and limestone rocky soils. It is often one of the first pioneer plant species to colonize newly disturbed areas. Altitudinal range: 0–1300 m.
Phenology: Flowering specimens of H. italicum subsp. siculum can be found from (May) June to August (September).
2c. Helichrysum italicum subsp. microphyllum (Willd.) Nyman, Consp. Fl. Eur. 1: 382 (1879).
≡ Gnaphalium microphyllum Willd., Sp. Pl. 3: 1863 (1803) [basionym]. ≡ Helichrysum microphyllum (Willd.) Cambess. in Mém. Mus. Hist. Nat. 14: 272 (1827). ≡ H. serotinum var. microphyllum (Willd.) Boiss., Voy. Bot. Espagne 2: 328 (1840). ≡ H. italicum var. microphyllum (Willd.) Boiss., Fl. Orient. 3: 234 (1875). ≡ H. angustifolium subsp. microphyllum (Willd.) Rouy, Fl. France 8: 195 (1903). ≡ H. stoechas f. microphyllum (Willd.) Knoche, Flora Balear. 2: 459 (1922).
Lectotype (designated by Galbany-Casals et al. (2006cGalbany-Casals, M., Sáez, L., Benedí, C. & Jarvis, C. E. 2006c. Typification of names in Gnaphalium L. and Helichrysum Mill. (Asteraceae), and some taxonomic notes. Taxon 55: 489–501. http://dx.doi.org/10.2307/25065597: 492): (B-W No. 15448-01 photo!).
Plants up to 40 cm high (Fig. 4B). Herbaceous parts of vegetative and flowering stems 3.2–22(38.4) cm, with abundant axillary leaf fascicles. Basal and medial leaves on vegetative and flowering stems 3–10(14.3) × 0.5–1.3 mm, linear to linear-lanceolate, margin revolute and often undulate, discolorous, subglabrous or rarely arachnoid and eglandular to sparsely glandular on the adaxial surface. Axillary leaves 1.5–3.7(5.7) × 0.6–1.1 mm. Synflorescence 6–23(35) × 8.5–27(37) mm, with 3–36(41) capitula. Capitula (3.9)4.3–6(6.5) × 2–3.5 mm, cylindrical to narrowly campanulate (Fig. 8C); involucral bracts 20–33(39) per capitulum (Fig. 8D); outermost bracts (0.7)1.1–2.4 × 0.4–0.9(1.2) mm, linear-lanceolate to lanceolate, with acute to subobtuse tips, arachnoid-tomentose to tomentose, eglandular to densely glandular; innermost bracts 3.4–5 × 0.5–1.3 mm, narrowly spatulate to linear, with obtuse rounded tips, glabrous to subglabrous and sparsely to densely glandular in the stereome. Florets (8)13–25 per capitulum; pistillate 1–6(8) per capitulum, corolla 2.3–3.5 mm; hermaphroditic (7)11–21 per capitulum, corolla 2.7–4 mm. Achenes ca. 0.9–1.1 × 0.4–0.7 mm, cylindrical to ovoid, with regularly scattered white duplex hairs. Pappus 2.2–3.5(3.9) mm.
Distribution and habitat: Helichrysum italicum subsp. microphyllum is endemic to Crete (Fig. 6). It is mostly found in mountain open shrubby formations. Altitudinal range 200–1600 m.
Phenology: Flowering specimens of H. italicum subsp. microphyllum can be found from (May) June to August (October).
2d. Helichrysum italicum subsp. tyrrhenicum (Bacch., Brullo & Giusso) Herrando, J. M. Blanco, L. Sáez & Galbany, comb. nov.
≡ Helichrysum microphyllum subsp. tyrrhenicum Bacch., Brullo & Giusso in Feddes Repert. 116(3–4): 272 (2005) [basionym].
Type: [Italy] Sardegna, Miniere di San Giovanni Binda (CA), 11.06.1998, Bacchetta & Brullo (holotype: CAT; isotypes: CAT, CAG, FI).
= Helichrysum argyreum Jord. & Fourr., Brev. Pl. Nov. 2: 68 (1868). ≡ H. angustifolium var. argyreum (Jord. & Fourr.) Rouy, Fl. France 8: 195 (1903).
= Helichrysum chloroticum Jord. & Fourr., Brev. Pl. Nov. 2: 68 (1868). ≡ H. angustifolium var. chloroticum (Jord. & Fourr.) Rouy, Fl. France 8: 195 (1903).
Plants up to 40 cm high (Figs. 4C, 4D and 5A). Herbaceous parts of vegetative and flowering stems 3.2–26(31.2) cm, with abundant axillary leaf fascicles. Basal and medial leaves on vegetative and flowering stems 3.7–20(29) × 0.5–1.7 mm, linear to linear-lanceolate, margin revolute and only rarely undulate, concolorous, arachnoid-tomentose to tomentose and sparsely glandular on the adaxial surface. Axillary leaves (1)1.5–6.6(8) × 0.4–1.5 mm. Synflorescence (6.2)8–47(69) × (9.3)14–37(51.5) mm, with 5–37(64) capitula. Capitula (3.5)4–6.5 × 2–3.5(4.6) mm, cylindrical to narrowly campanulate (Fig. 8E); involucral bracts 18–40 per capitulum (Fig. 8F); outermost bracts 1–2.5 × 0.4–1.5(1.8) mm, linear-lanceolate to lanceolate, with obtuse to acute tips, arachnoid-tomentose to tomentose, eglandular to densely glandular; innermost bracts (2.5)3.1–5 × 0.3–1.3 mm, linear, narrowly lanceolate, with acute, obtuse or rounded tips, glabrous to arachnoid, rarely arachnoid-tomentose and sparsely to densely glandular in the stereome. Florets 13–36(39) per capitulum; pistillate 3–10(12) per capitulum, corolla 2–4 mm; hermaphroditic 9–29 per capitulum, corolla (2.5)2.8–4.4 mm. Achenes 0.6–1 × 0.2–0.6 mm, cylindrical to ovoid, with regularly scattered white duplex hairs, sometimes glabrous. Pappus (2.1)2.4–3.9(4.1) mm.
Distribution and habitat: Helichrysum italicum subsp. tyrrhenicum has a disjunct distribution area between the Mediterranean islands of Corsica, Sardinia, Majorca and Dragonera islet (Fig. 6). It occupies wide and diverse open habitats, including road banks and path margins, mountain open shrubby formations in rocky substrates, maritime rocks and sand dunes. In particular, Majorcan populations of H. italicum subsp. tyrrhenicum are restricted to coastal areas. Altitudinal range: 0–900 m.
Phenology: Flowering specimens of H. italicum subsp. tyrrhenicum can be found from (May) June to August (October).
ACKNOWLEDGEMENTSTop
We are very grateful to the curators of the herbaria that provided material and images: BC, BCN, FI, G, MA and MPU. We thank S. Arrabal, E. Blanco and G. Blanco for providing helpful, enthusiastic and pleasant assistance in field collections. We also thank J. Fuertes, T. Garnatje, J. López, J. Molero, A. Martínez Abraín, M. Mus, G. Nieto and J. Rosselló for providing material from their own field collections. L. Hugot and D. Jeanmonod provided advice for collections in Corsica. S. Giralt provided Latin advice for the H. massanellanum epithet selection. R. D. Smissen, C. Aedo and E. Rico provided helpful comments on the manuscript. This work was partly financed by the Spanish government (CGL2007-60781/BOS, CGL2009-13322-C03-03/BOS, CGL2010-18631/BOS) and by the Catalan government (Ajuts a grups consolidats 2014-SGR 514).
REFERENCESTop
|
| 1. |
Anderberg, A. A. 1991. Taxonomy and phylogeny of the tribe Gnaphalieae (Asteraceae). Opera Botanica 104: 1–195. |
|
| 2. |
Angiolini, C., Bacchetta, G., Brullo, S., Casti, M., Giusso del Galdo, G. & Guarino, R. 2005. The vegetation of mining dumps in SW-Sardinia. Feddes Repertorium 116: 243–276. http://dx.doi.org/10.1002/fedr.200411072 |
|
| 3. |
Boissier, E. 1840. Voyage botanique dans le midi de l’Espagne pendant l’année 1837 2. Gide et Cie, Paris. |
|
| 4. |
Boissier, E. 1875. Flora orientalis sive enumeratio plantarum in Oriente a Graecia et Aegypto and Indiae fines hucusque observatarum 3. H. Georg, Basileae & Genevae. |
|
| 5. |
Bonafè, F. 1980. Flora de Mallorca 4. Ed. Moll, Palma de Mallorca. |
|
| 6. |
Cambessèdes, J. 1827. Enumeratio plantarum quas in insulis Balearibus collegit J. Cambèssedes. Mémoires du Muséum d’Histoire Naturelle. Paris 14: 173–335. |
|
| 7. |
Duvigneaud, J. 1979. Catalogue provisoire de la flore des Baléares (2nd ed.). Bulletin de la Société pour l’Échange des Plantes Vasculaires de l’Europe et du Bassin Méditerranéen 17: 1–43. |
|
| 8. |
Galbany-Casals, M., Blanco-Moreno, J. M., Garcia-Jacas, N., Breitwieser, I. & Smissen, R. D. 2011. Genetic and morphological variation in the Mediterranean Helichrysum italicum subsp. microphyllum (Asteraceae; Gnaphalieae). Plant Biology 13: 678–687. http://dx.doi.org/10.1111/j.1438-8677.2010.00411.x |
|
| 9. |
Galbany-Casals, M., Garcia-Jacas, N., Sáez, L., Benedí, C. & Susanna, A. 2009. Systematics, biogeography and character evolution in Mediterranean, Asiatic and Macaronesian Helichrysum (Asteraceae, Gnaphalieae) inferred from nuclear phylogenetic analyses. International Journal of Plant Sciences 170: 365–380. http://dx.doi.org/10.1086/596332 |
|
| 10. |
Galbany-Casals, M., Sáez, L. & Benedí, C. 2006a. A taxonomic revision of Helichrysum Mill. sect. Stoechadina (DC.) Gren. & Godr. (Asteraceae, Gnaphalieae). Canadian Journal of Botany 84: 1203–1232. http://dx.doi.org/10.1139/b06-082 |
|
| 11. |
Galbany-Casals, M., Sáez, L. & Benedí, C. 2006b. Conspectus of Helichrysum Mill. Sect. Stoechadina (DC.) Gren & Godr. (Asteraceae, Gnaphalieae). Orsis 21: 58–81. |
|
| 12. |
Galbany-Casals, M., Sáez, L., Benedí, C. & Jarvis, C. E. 2006c. Typification of names in Gnaphalium L. and Helichrysum Mill. (Asteraceae), and some taxonomic notes. Taxon 55: 489–501. http://dx.doi.org/10.2307/25065597 |
|
| 13. |
Georgiadou, E. 1985. Helichrysum. In: Meikle, R. D. (Ed.), Flora of Cyprus 2. Royal Botanic Gardens, Kew: 888–889. |
|
| 14. |
Greuter, W. 2008. Helichrysum Mill. In: Greuter, W. & Raab-Straube, E. von (Eds.), Med-checklist 2 Dicotyledones (Compositae). Organisation for the Phyto-Taxonomic Investigation of the Mediterranean Area (OPTIMA), Genève: 234–239. |
|
| 15. |
Hilliard, O. M. 1983. Helichrysum Mill. In: Leistner, O. A. (Ed.), Flora of Southern Africa 33 (7, 2). Department of Agriculture, Pretoria: 61–310. |
|
| 16. |
Holmboe, J. 1914. Studies on the vegetation of Cyprus: Based upon researches during the spring and summer 1905. John Griegs, Bergen. |
|
| 17. |
IUCN (International Union for Conservation of Nature) 2001. IUCN Red List categories and criteria Version 3.1. IUCN Species Survival Commission, Gland,& Cambridge. |
|
| 18. |
Jeanmonod, D. 1998. Xanthium subg. Xanthium et Helichrysum italicum deux cas taxonomiques ardus. Candollea 53: 435–457. |
|
| 19. |
Knoche, H. 1922. Flora Balearica. Etude phytogéographique sur les îles Baléares 2. Imp. Roumégous et Déhen, Montpellier. |
|
| 20. |
López-Pujol, J., Martinell, M. C., Massó, S., Blanché, C. & Sáez, L. 2013. The ‘paradigm of extremes’: extremely low genetic diversity in an extremely narrow endemic species, Coristospermum huteri (Umbelliferae). Plant Systematics and Evolution 299: 273–275. http://dx.doi.org/10.1007/s00606-012-0732-3 |
|
| 21. |
Marès, P. & Vigineix, G. 1880. Catalogue raisonné des plantes vasculaires des îles Baléares. Ed. G. Masson, Paris. |
|
| 22. |
Médail, F. & Diadema, K. 2009. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. Journal of Biogeography 36: 1333–1345. http://dx.doi.org/10.1111/j.1365-2699.2008.02051.x |
|
| 23. |
Médail, F. & Quézel, P. 1997. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean Basin. Annals of the Missouri Botanical Garden 84: 112–127. http://dx.doi.org/10.2307/2399957 |
|
| 24. |
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A. & Kent, J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. http://dx.doi.org/10.1038/35002501 |
|
| 25. |
Pla, V., Sastre, B. & Llorens, L. 1992. Aproximació al catàleg de la flora vascular de les illes Balears: 1992. Universitat de les Illes Balears, Palma. |
|
| 26. |
Sáez, L. 2010. Plantes endèmiques dels Països Catalans. In: Giralt, J. (Ed.), Història natural dels Països Catalans, Suplement fauna i flora. Enciclopèdia Catalana, Barcelona: 161–164. |
|
| 27. |
Sáez, L., Fraga, P. & López-Alvarado, J. 2013. The flora of the Balearic Islands. In: Cardona, E., Estaún, I., Comas, M. & Fraga, P. (Eds.), Islands and plants: preservation and understanding of flora on Mediterranean Islands. Consell Insular de Menorca, Maó: 91–103. |
|
| 28. |
Sáez, L. & Rosselló, J. A. 2001. Llibre Vermell de la flora amenaçada de les IIles Balears. Conselleria de Medi Ambient (Govern de les Illes Balears), Palma de Mallorca. |
|
| 29. |
Willdenow, C. L. 1803. Caroli a Linné Species Plantarum [...] editio quarta [...], 3(3). Impensis G. C. Nauk, Berolini. |
|
Appendix 1. List of all specimens examined and used in analyses. Top
Helichrysum italicum (Roth) G. Don subsp. italicum: Croatia, Istria: in arenosis ad Polam, 20 m, solo calcareo, s. d., Neugebauer s. n. (BC 30988). Cyprus: Mont Troodos, 5000-6400’ [1500-1950 m], 20.07.1912, Haradjian 527 (G); Kokkinovonno [sic], 2900’ [884 m], 06.07.1937, Kennedy 959 (G); a 10 km abans d’arribar al poble de Troodos des del S, 34º53’32,8’’N 32º51’46,2’’E, 1230 m, marges assolellats de la carretera, clarianes de pinedes, 31.07.2008, Galbany 2036 et al. (BC 948594); entre Kakopetria i Agios Nikolaos, molt aprop de Kakopetria, 34º59’2,6’’N 32º53’59,3’’E, 744 m, marges rocosos de la carretera, 31.07.2008, Galbany 2038 et al. (BC 948593); a 2 km al S de Kampos, 35º1’10,4’’N 32º43’32,6’’E, 862 m, marge descobert de la carretera, 01.08.2008, Galbany 2042 et al. (BC 949110). France, Corsica: Bastia, 10.07.1868, Debeaux s. n. (BC 30896); Calvi, pointe de la Revellata, 10 m, pelouse sublittorale sur sol granitique, sur la côte N de la pointe, 22.06.1976, Lambinon 76/co/546 & Duvigneaud (BC 629912); Haute Corse, Barbaggiu, Bocca di Teghime, 530 m, herbazales subnitrófilos, 05.08.1996, Serra 4609 & Bort (MA 623431); carretera D48 que va de Sartène a Tizzano, 41º32’23’’N 8º51’49,9’’E, 34 m, marge de carretera saulonós, 07.08.2008, Galbany 2063 & Arrabal (BC 948733); carretera D48 que va de Sartène a Tizzano, 41º32’47,2’’N 8º53’0,5’’E, 42 m, marge de la carretera, 07.08.2008, Galbany 2064 & Arrabal (BC 948732); aprox. 1 km abans d’arribar al Col de Bavella des de Zonza, 41º47’32,4’’N 9º13’18,8’’E, 1192 m, clariana de pineda, sòl rocós Si, 08.08.2008, Galbany 2065 & Arrabal (BC 948730); voltants de Monticello a 1 km anant cap a Ficajola, 42º36’59,2’’N 8º58’15,4’’E, 198 m, vessant rocós, 09.08.2008, Galbany 2069 & Arrabal (BC 948728); voltants de Bigorno a uns 500 m cap a Lento, 42º31’52,8’’N 9º17’45,7’’E, 661 m, vessant rocós, 10.08.2008, Galbany 2072 & Arrabal (BC 948725); Corti, Vall de Restonica a uns 500 m abans d’arribar al pàrquing Bergeries, 42º14’22’’N 9º2’9,2’’E, 1300 m, vessant rocós, 11.08.2008, Galbany 2073 & Arrabal (BC 948724); voltants de Figari, aprox. a 1 km de Figari per la carretera que va a l’aeroport, 41º30’1,5’’N 9º6’57’’E, 23 m, marges de la carretera, clarianes de màquia, 12.08.2008, Galbany 2074 & Arrabal (BC 948706). Greece, Ikaria: 02.08.1887, Major 934 (G). Naxos: afores de Cora, 37º05’34,9’’N 25º22’23,9’’E, 1-2 m, dunes marítimes, 20.07.2008, Galbany 2001 et al. (BC 948659); Mont Zeus, a prop de la font, al final de la carretera que puja cap al cim des de prop de Filoti, 37º02’13,8’’N 25º29’33,4’’E, 499 m, phrygana, 20.07.2008, Galbany 2003 et al. (BC 948656). Syros: Syros, 05.1871, Orphanides 1144 (G). Italy: Rapolano, près Sienne, lieux incultes arides, 08.1873, Sommier s. n. (BC 30886); Massa e Carrara, 07.07.1886, [illeg.] s. n. (BC 30902); incontro Firenze, luoghi aridi Sasfosi, 1902, Tani s. n. (FI); Vesuvio, 200-300 m, 20.06.1912, Pellanda s. n. (BC 30997); Apulia Tarentum, loco dicto Gravina di Leucaspide, 30 m, in alveo glareoso, rarissime inundato, 14.06.1914, Lacaita s. n. (BC 30990); Sila, in ditione Longobucco, loco Ponte di Cecita dicto, 1100 m, solo granitico, 03.08.1918, Fiori s. n. (BC 30991); Monterosso al Mare, La Spezia, 09.06.1927, [illeg.] s. n. (BC 75937); in glareosis prope Basento flumen, 04.08.1927, Gavioli s. n. (FI); Retignano, 16.08.1942, Corradi s. n. (MA 186294); Montepulciano, colline argillose della Val d’Orcia, 300-600 m, 05.08.1958, Fabbri s. n. (FI). Elba: Marciana, 550 m, 22.06.1968, Geissler 6252 (G 217797). Pianosa: dal paese a Cala S. Giovanni, 21.05.1998, Baldini s. n. (FI). Montenegro: Budva, in autocampo Cruena Glavica in proximitate oppiduli Sveti Stefan, 50–100 m, sparso in fruticetis sempervirentibus et in saxosis regionis mediterraneae inferioris, 07.07.1982, Černoch 39169 (BC 657165, G 293376). Morocco: Guelb-er-Rahal, 1500–2000 m, in glareosis torrentum et in pascuis lapidosis calcareis inter querceta, 28.06.1927, Maire s. n. (MPU); Demnate, au dessus d’Imi-n-Ifri, 25.07.1941, Lhermite 14 (MPU). Helichrysum italicum (Roth) G. Don subsp. microphyllum (Willd.) Nyman: Greece, Crete: Distr. Hierapetra, Steinige Abhänge in der Bergregion des Aphendi Kavusi, 03.08.1904, Dörfler 1036 (G); Sphakia, in saxosis subalpinis montis Theodoros, 14.07.1935, Skřivanek s. n. (MPU); supra Psychro Lasithi, 1000 m, in rupestribus, 18.07.1939, Regel s. n. (G); passat el poble d’Imbros, a 1 Km en direcció Sphakia, 15.08.2002, Garnatje 121 & Luque (BCN 20564); Sitia, a 2 km de l’entrada de Tripti des de Kato Horio, 18.08.2002, Garnatje 141 & Luque (BCN 20742); Sitia, camí de Messa Mouliana a Oros Koprokefala, 18.08.2002, Garnatje 145 & Luque (BCN 20575); Sitia, pujant al Mt. Afenthis, 19.08.2002, Garnatje 150 & Luque (BCN 20739); Sitia, pujant al Mt. Afenthis, 19.08.2002, Garnatje 152 & Luque (BCN 20741); Sitia, pujant al Mt. Afenthis, 19.08.2002, Garnatje 153 & Luque (BCN 20740); Sitia, pujant al Mt. Afenthis, 19.08.2002, Garnatje 154 & Luque (BCN 20574); Sitia, pujant al Mt. Afenthis, 19.08.2002, Garnatje 155 & Luque (BCN 20712); Altiplà de Lasithi, entre Mesa Lasithi i Mesa Potami, 35º12’6,7’’N 25º31’10,3’’E, 1022 m, phrygana esclarissada, 23.07.2008, Galbany 2006 et al. (BC 948656); entre Ano Khorio i Thripti, 35º04’39,1’’N 25º50’21,3’’E, 608 m, phrygana, 24.07.2008, Galbany 2009 et al. (BC 948650); arribant a Omalos des de Lakki, a 5 km d’Omalos, 35º21’51,1’’N 23º54’41’’E, 980 m, marges i clarianes en bosc de Cupressus sempervirens subsp. horizontalis, 27.08.2008, Galbany 2026 et al. (BC 948653). Helichrysum italicum (Roth) G. Don subsp. siculum (Jord. & Fourr.) Galbany, L. Sáez & Benedí: Italy, Sicily: Catania, Mt. Etna, prop de Nicolosi, pioner sobre terreny volcànic, 16.09.2001, Galbany s. n. (BCN 24030, BCN 25248, BCN 34262); P. R. Nebrodi, pr. Galati Mamertino, Catafurcu, 800 m, brolles calcícoles, 21.09.2001, Galbany s. n. (BCN 6116, BCN 24031, BCN 25234). Helichrysum italicum (Roth) G. Don subsp. tyrrhenicum (Bacch., Brullo & Giusso) Herrando, J. M. Blanco, L. Sáez & Galbany: France, Corsica: Bonifacio, les plages, 12.07.1880, Reverchon 305 (MPU); Bonifacio, au bord de la falaise, à côté (nord-nord-ouest) du sémaphore de Pertusato, 105 m, sommet de la falaise calcaire, sur sol sableux, enbordure d’une formation de garrigue très basse, 18.05.1983, Thiébaud 4464 & Roguet (BC 657164); Golfe de Santa Manza, plage de Maora, groupement pionnier, sur plage de sable grossier, 02.06.1997, Lambinon 97/Co/117 & Van den Sande (MA 595752); Bonifacio, Cabo Pertusato, cra. a Playa Piantarella, maquia litoral muy venteada, 06.06.2002, Nieto 4506 & Fuertes (BCN 20713); La Tonnara, 41º25’35,8’’N 9º6’51,8’’E, 41 m, clarianes de màquia de Pistacia lentiscus i Juniperus phoenicea, 06.08.2008, Galbany 2050 & Arrabal (BC 948624); entre Semaphore de Pertusato i el Far de Pertusato, 78 m, clarianes de màquia de Pistacia lentiscus i Juniperus phoenicea, sobre el penyasegat, 06.08.2008, Galbany 2052 & Arrabal (BC 949617); Golfu di Sant’Amanza, per la carretera de Gurgazu cap al NE, 41º24’55,3’’N 9º14’11,4’’E, 3 m, clarianes de màquia de Pistacia lentiscus i Juniperus phoenicea, 06.08.2008, Galbany 2053 & Arrabal (BC 948628); platja dels voltants de Roccapina, que no té carretera per accedir-hi, 41º29’30,5’’N 8º57’41,4’’E, 3 m, roques de la platja, 07.08.2008, Galbany 2058 & Arrabal (BC 948626); Cap Corse, Barcaggio, 43º0’27,9’’N 9º23’59,1’’E, 13 m, roques marítimes, 10.08.2008, Galbany 2070 & Arrabal (BC 948625); Ile Lavezzi, 41º20’14’’N 9º15’35,7’’E, 4 m, entre roques, 12.08.2008, Galbany 2075 & Arrabal (BC 948623). Italy, Sardinia: Santa Teresa Gallura, plages calcaires, 08.07.1881, Reverchon 96 (MPU); Isola Maddalena, 06.1893, Vaccari s. n. (BC 30993); in aridis sterilibus prope Sassari, 1895, Nicotra 3529 (G); San Giovanni Suegiu [sic], sorrals platja, 15.04.1973, Folch et al. s. n. (BC 656457); Carloforte, La Punta, extrémité nord de l’île de San Pietro, 10 m, lieux arides pierreux, 31.05.1988, Marchi et al. s. n. (G 397480); playa de Porto Liscia, arenal estabilizado en lentiscar - sabinar, 07.06.2002, Nieto 4529 & Fuertes (BCN 20718); NW Oristano, Capo Manu, entre el cabo y Su Pallesu, 07.06.2002, Nieto & Fuertes s. n. (BCN 20711); entre Porto Torres i Castelsardo, dunes de la platja Platamone, 29.06.2002, Galbany & Sáez s. n. (BCN 20738, BCN 25235); Monte Scuine, pr. Baunei, en vessant rocós Ca., 30.06.2002, Galbany & L. Sáez s. n. (BCN 20576); Tempio, per carretera d’ascenció al Mt. Limbara, a uns 900 m, 30.06.2002, Galbany & Sáez s. n. (BCN 20578, 2 specimens); Capo Carbonara, 01.07.2002, Galbany & Sáez s. n. (BCN 20709); Capo Falcone, 03.06.2003, Molero & López s. n. (BCN 20708); platja de Porto Liscia, 41º11’30,1’’N 9º17’46,3’’E, 1 m, dunes marítimes, 13.08.2008, Galbany 2076 & Arrabal (BC 948615). Spain, Dragonera: Dragonera, 27.07.1991, Mus s. n. (herb. Universitat Illes Balears, 4 specimens); pujant a Sa Pòpia, en màquia oberta, sobre terreny rocós, 20.06.2001, Galbany & Sáez s. n. (BCN 20717). Majorca: Port d’Andratx, Cala Moragues, 03.07.1991, Mus s. n. (herb. Universitat Illes Balears, 4 specimens); Cap des Llamp, 03.07.1991, Mus s. n. (herb. Universitat Illes Balears); Penyes Rotges, 27.07.1991, Mus s. n. (herb. Universitat Illes Balears, 5 specimens); S’Hort de Sa Cova, Valdemossa, 31SDD6295, 10 m, matollars litorals sobre gresos silicis del Bundsandstein i conglomerats quaternaris, 01.07.2013, Martínez s. n. (BC 949116, BC 949115, BC 948602, BC 948611, BC 948614). Helichrysum massanellanum Herrando, J. M. Blanco, L. Sáez & Galbany: Spain, Majorca: Massanella, 01.07.1951, Cañigueral s. n. (BC 118706); Puig de Massanella, supra Coma Freda, 1200 m, exp. N, in Teucrieto subspinosi, 12.07.1956, A. & O. de Bolòs s. n. (BC 137143); Massanella, 1974, Lesonef s. n. (MA 620096); Puig de Massanella, 1200 m, matorrales sobre calizas, 25.09.1986, Romo 3562 (BC 659149); Massanella, 02.08.1991, Mus et al. s. n. (herb. Universitat Illes Balears, 5 specimens); Coll de Ses Cases de Neu, c. Coll des Telègraf, 1205 m, Ca., 21.06.2001, Galbany & Sáez s. n. (BCN 6115, BCN 20580 holotype, BC isotype); c. Coll des Telègraf, 990 m, Ca., 21.06.2001, Galbany & Sáez s. n. (BCN 20714, BCN 25228); Coll des Prat, 07.2009, Sáez s. n. (BC 949117); Coma des Nevaters, c. Coll des Telègraf, 1100 m, talussos dolomítics, 08.2001, Sáez 5739 (BCN 20710).
Appendix 2. Top
Pooled within-groups correlations between discriminating variables
and standardized
canonical discriminant functions. Variables ordered by absolute size
of correlation within function |
| Dimension 1 |
Correlation between variable and function |
| Caulinar leaf length (mm) |
0.355 |
| Leaf margin undulation |
−0.363 |
| Leaf margin undulation |
−0.363 |
| Caulinar leaf length / caulinar leaf width |
0.342 |
| Synflorescence width (mm) |
0.332 |
| Number of capitula per synflorescence |
0.279 |
| Synflorescence length (mm) |
0.222 |
| Outermost involucral bract lenght (mm) |
−0.198 |
| Eglandular indumentum of innermost involucral bract abaxial side (external) |
0.174 |
| Capitulum lenght (mm) |
−0.171 |
| Innermost involucral bract lenght (mm) |
−0.145 |
| Outermost involucral bract lenght / outermost involucral bract width |
−0.143 |
| Medial innermost involucral bract lenght / medial outermost involucral bract lenght |
0.137 |
| Presence/Absence of axillary leaf fascicles |
0.122 |
| Capitulum width (mm) |
−0.113 |
| Glandular indumentum of leaf abaxial side |
0.112 |
| Number of hermaphroditic florets per capitulum |
−0.110 |
| Number of pistillate florets per capitulum |
0.105 |
| Glandular indumentum of outermost involucral bract abaxial side (external) |
−0.089 |
| Innermost involucral bract width (mm) |
−0.089 |
| Outermost involucral bract consistency |
−0.083 |
| Eglandular indumentum of leaf adaxial side |
0.068 |
| Eglandular indumentum of outermost involucral bract abaxial side (external) |
−0.056 |
| Total number of florets per capitulum |
−0.048 |
| Outermost involucral bract width (mm) |
0.031 |
| Number of involucral bracts per capitulum |
−0.030 |
| Leaf margin (flat or revolute) |
−0.028 |
| Caulinar leaf width |
0.023 |
| Glandular indumentum of innermost involucral bract abaxial side (external) |
0.017 |
| Innermost involucral bract length / innermost involucral bract width |
−0.016 |
| Capitulum lenght / capitulum width |
0.011 |
| Dimension 2 |
Correlation between variable and function |
| Eglandular indumentum of leaf adaxial side |
0.478 |
| Medial innermost involucral bract lenght / medial outermost involucral bract lenght |
−0.314 |
| Caulinar leaf length / caulinar leaf width |
−0.242 |
| Innermost involucral bract lenght (mm) |
−0.176 |
| Caulinar leaf width |
−0.170 |
| Presence/Absence of axillary leaf fascicles |
0.167 |
| Innermost involucral bract width (mm) |
−0.151 |
| Outermost involucral bract lenght (mm) |
0.143 |
| Number of involucral bracts per capitulum |
−0.139 |
| Eglandular indumentum of innermost involucral bract abaxial side (external) |
−0.130 |
| Glandular indumentum of innermost involucral bract abaxial side (external) |
0.114 |
| Glandular indumentum of leaf abaxial side |
−0.097 |
| Capitulum lenght (mm) |
−0.096 |
| Number of pistillate florets per capitulum |
0.094 |
| Leaf margin (flat or revolute) |
−0.079 |
| Leaf margin undulation |
0.079 |
| Capitulum width (mm) |
−0.074 |
| Eglandular indumentum of outermost involucral bract abaxial side (external) |
0.073 |
| Outermost involucral bract lenght / outermost involucral bract width |
0.070 |
| Outermost involucral bract width (mm) |
0.062 |
| Synflorescence width (mm) |
0.052 |
| Innermost involucral bract length / innermost involucral bract width |
0.048 |
| Number of hermaphroditic florets per capitulum |
−0.037 |
| Synflorescence length (mm) |
0.031 |
| Capitulum lenght / capitulum width |
0.029 |
| Number of capitula per synflorescence |
−0.015 |
| Total number of florets per capitulum |
0.008 |
| Outermost involucral bract consistency |
0.002 |
| Glandular indumentum of outermost involucral bract abaxial side (external) |
0.000 |
|
Appendix 3. Top
Predicted group membership obtained from tabulating the CDA predicted membership
against the a priori assignment, expressed as total number of individuals and,
between
parentheses, as percentage over row totals. |
| Predicted Group Membership |
| Taxon |
H. italicum subsp. italicum |
H. italicum subsp. siculum |
H. italicum subsp. microphyllum |
H. italicum subsp. tyrrhenicum |
H. massanellanum |
| H. italicum subsp. italicum |
36 (94.7) |
0 |
0 |
2 (5.3) |
0 |
| H. italicum subsp. siculum |
0 |
6 (100) |
0 |
0 |
0 |
| H. italicum subsp. microphyllum |
0 |
0 |
14 (100) |
0 |
0 |
| H. italicum subsp. tyrrhenicum |
1 (2.2) |
0 |
2 (4.3) |
43 (93.5) |
0 |
| H. massanellanum |
0 |
0 |
0 |
0 |
15(100) |
|
Appendix 4. Top
Descriptive statistics of all characters studied for each taxon, and results
from
the overall test for difference between groups (p-value) and of pairwise
comparisons
of means (post-hoc tests). Only significant differences found in the pairwise
comparisons
are reported. X = mean value; SD = standard deviation; Min = minimum
value;
Max = maximum value. Taxa abbreviations and number of studied
specimens (N):
ita = H. italicum subsp. italicum (N = 38); sic = H. italicum
subsp. siculum
(N = 6); mic = H. italicum subsp. microphyllum (N = 14);
tyr = H. italicum subsp. tyrrhenicum (N = 46); mas = H. massanellanum (N = 15). |
|
Character
|
Taxon
|
X
|
SD
|
Min
|
Max
|
p-value
|
Significant differences in post-hoc test
|
|
1. Presence (1) / absence (0) of axillary leaf fascicles1
|
ita
|
0.43
|
0.49
|
0
|
1
|
0.000
|
ita < sic P = 0.024
ita < mic P = 0.000
ita < tyr P = 0.000
mas < ita P = 0.009
mas < sic P = 0.000
mas < mic P = 0.000
mas < tyr P = 0.000
|
|
sic
|
0.91
|
0.20
|
0.5
|
1
|
|
mic
|
0.96
|
0.13
|
0.5
|
1
|
|
tyr
|
0.78
|
0.29
|
0
|
1
|
|
mas
|
0.06
|
0.25
|
0
|
0.5
|
|
2. Caulinar leaf length (mm)2
|
ita
|
18.60
|
6.04
|
8
|
37
|
0.000
|
ita > mic P = 0.000
ita > tyr P = 0.000
sic > mic P = 0.028
mas < tyr P = 0.000
mas < ita P = 0.000
mas < sic P = 0.000
|
|
sic
|
13.80
|
2.79
|
7
|
21
|
|
mic
|
7.46
|
2.84
|
3
|
14.3
|
|
tyr
|
10.42
|
3.76
|
3.7
|
29
|
|
mas
|
4.77
|
1.09
|
2
|
10
|
|
3. Caulinar leaf width (mm)2
|
ita
|
0.91
|
0.12
|
0.4
|
1.8
|
0.001
|
tyr > ita P = 0.005
tyr > mas P = 0.013
|
|
sic
|
0.88
|
0.07
|
0.6
|
1.1
|
|
mic
|
0.92
|
0.14
|
0.5
|
1.3
|
|
tyr
|
1
|
0.12
|
0.5
|
1.7
|
|
mas
|
0.89
|
0.08
|
0.5
|
1.3
|
|
4. Caulinar leaf length / caulinar leaf width2
|
ita
|
21.47
|
8.01
|
7.22
|
51.67
|
0.000
|
ita > mic P = 0.000
ita > tyr P = 0.000
sic > mic P = 0.023
mas < sic P = 0.001
mas < ita P = 0.000
mas < tyr P = 0.019
|
|
sic
|
16.00
|
2.52
|
9
|
33.33
|
|
mic
|
8.25
|
3.14
|
2.46
|
16.25
|
|
tyr
|
10.37
|
3.35
|
3.7
|
29
|
|
mas
|
5.49
|
1.38
|
2
|
13.33
|
|
5. Leaf margin (flat or revolute). 0: all leaves flat; 1: most leaves flat, some revolute; 2: flat and revolute leaves in the same proportion; 3: most leaves revolute, some flat; 4: all leaves revolute1
|
ita
|
3.15
|
0.49
|
2
|
4
|
>0.05
|
|
|
sic
|
3.33
|
0.51
|
3
|
4
|
|
mic
|
3.28
|
0.46
|
3
|
4
|
|
tyr
|
3.10
|
0.31
|
3
|
4
|
|
mas
|
3.26
|
0.45
|
3
|
4
|
|
6. Leaf margin undulation. 0: most leaves without undulate margins; 0.5: some leaves undulate; 1: most leaves with undulate margins1
|
ita
|
0
|
0
|
0
|
0
|
0.000
|
ita < tyr P = 0.000
mic > ita P = 0.000
mic > sic P = 0.000
mic >tyr P = 0.000
mas > ita P = 0.000
mas > sic P = 0.000
mas > tyr P = 0.000
|
|
sic
|
0
|
0
|
0
|
0
|
|
mic
|
0.82
|
0.37
|
0
|
1
|
|
tyr
|
0.34
|
0.45
|
0
|
1
|
|
mas
|
0.96
|
0.12
|
0.5
|
1
|
|
7. Eglandular indumentum of leaf adaxial side. 0: 0–5% coverage; 1: 6–25% coverage; 2: 26–50% coverage; 3: 51–75% coverage; 4: 76–100% coverage2
|
ita
|
2.47
|
0.73
|
1
|
3.5
|
0.000
|
sic < ita P = 0.00
sic < mic P = 0.020
sic < tyr P = 0.000
tyr > ita P = 0.000
tyr > mic P = 0.000
tyr > mas P = 0.000
|
|
sic
|
1.25
|
0.52
|
0.5
|
2
|
|
mic
|
2.35
|
0.74
|
1
|
3
|
|
tyr
|
3.20
|
0.56
|
2
|
4
|
|
mas
|
2.00
|
0.53
|
1
|
3
|
|
8. Glandular indumentum of leaf abaxial side.0: 0–5% coverage; 1: 6–25% coverage; 2: 26–50% coverage; 3: 51–75% coverage; 4: 76–100% coverage2
|
ita
|
2.34
|
0.78
|
0
|
3
|
0.004
|
ita > tyr P = 0.031
ita > mas P = 0.015
|
|
sic
|
2.33
|
0.40
|
2
|
3
|
|
mic
|
1.75
|
0.97
|
0
|
3
|
|
tyr
|
1.84
|
0.78
|
0.5
|
3
|
|
mas
|
1.60
|
0.47
|
1
|
2
|
|
9. Synflorescence length (mm)3
|
ita
|
21.06
|
5.13
|
10
|
62
|
0.000
|
ita > mic P = 0.000
ita > tyr P = 0.005
ita > mas P = 0.000
tyr > mic P = 0.005
tyr > mas P = 0.000
mas < sic P = 0.016
|
|
sic
|
15.75
|
3.41
|
6
|
25
|
|
mic
|
11.22
|
4.32
|
6
|
35
|
|
tyr
|
17.18
|
6.62
|
6.2
|
69
|
|
mas
|
10.22
|
4.04
|
4.5
|
32
|
|
10. Synflorescence width (mm)2
|
ita
|
30.01
|
6.91
|
15
|
80
|
0.000
|
ita > sic P = 0.000
ita > mic P = 0.000
ita > tyr P = 0.000
ita > mas P = 0.000
tyr > mic P = 0.007
tyr > mas P = 0.000
|
|
sic
|
19.20
|
2.51
|
12
|
26
|
|
mic
|
16.45
|
5.07
|
8.5
|
37
|
|
tyr
|
23.00
|
6.44
|
9.3
|
51.5
|
|
mas
|
11.71
|
3.10
|
3
|
21
|
|
11. Number of capitula per synflorescence3
|
ita
|
33.48
|
16.57
|
8
|
120
|
0.000
|
ita > sic P = 0.001
ita > mic P = 0.000
ita > tyr P = 0.000
ita > mas P = 0.000
tyr > mic P = 0.042
mas < sic P = 0.000
mas < mic P = 0.000
mas < tyr P = 0.000
|
|
sic
|
14.33
|
4.48
|
3
|
42
|
|
mic
|
13.40
|
7.06
|
3
|
41
|
|
tyr
|
18.94
|
8.31
|
5
|
64
|
|
mas
|
4.31
|
2.67
|
1
|
18
|
|
12. Capitulum length (mm)2
|
ita
|
4.99
|
0.44
|
4
|
6.5
|
0.000
|
mas > ita P = 0.000
mas > mic P = 0.000
mas > tyr P = 0.000
|
|
sic
|
5.31
|
0.49
|
4
|
6
|
|
mic
|
4.92
|
0.36
|
3.9
|
6.5
|
|
tyr
|
5.09
|
0.40
|
3.5
|
6.5
|
|
mas
|
5.69
|
0.41
|
4
|
7
|
|
13. Capitulum width (mm)2
|
ita
|
2.87
|
0.33
|
2
|
4
|
0.004
|
mas > ita P = 0.003
mas > mic P = 0.026
mas > tyr P = 0.013
|
|
sic
|
3.16
|
0.45
|
2.2
|
4
|
|
mic
|
2.87
|
0.29
|
2
|
3.5
|
|
tyr
|
2.95
|
0.47
|
2
|
4.6
|
|
mas
|
3.34
|
0.43
|
2.5
|
5
|
|
14. Capitulum length / capitulum width2
|
ita
|
1.76
|
0.23
|
1.28
|
2.75
|
>0.05
|
|
|
sic
|
1.71
|
0.28
|
1.14
|
2.27
|
|
mic
|
1.73
|
0.19
|
1.28
|
2.5
|
|
tyr
|
1.76
|
0.26
|
1.1
|
2.56
|
|
mas
|
1.73
|
0.23
|
1.1
|
2.48
|
|
15. Number of hermaphroditic florets per capitulum2
|
ita
|
15.37
|
2.99
|
9
|
26
|
0.000
|
mas > ita P = 0.006
mas > mic P = 0.001
mas > tyr P = 0.010
|
|
sic
|
15.75
|
2.29
|
11
|
20
|
|
mic
|
14.07
|
2.56
|
7
|
21
|
|
tyr
|
15.73
|
3.85
|
9
|
29
|
|
mas
|
18.86
|
2.80
|
11
|
26
|
|
16. Number of pistillate florets per capitulum2
|
ita
|
6.03
|
1.54
|
2
|
10
|
0.002
|
ita > mic P = 0.000
ita > mas P = 0.037
tyr > mic P = 0.000
|
|
sic
|
4.91
|
0.96
|
3
|
8
|
|
mic
|
3.79
|
1.21
|
1
|
8
|
|
tyr
|
5.86
|
1.51
|
3
|
12
|
|
mas
|
4.68
|
1.98
|
0
|
10
|
|
17. Total number of florets per capitulum2
|
ita
|
21.40
|
3.89
|
14
|
34
|
0.008
|
mic < tyr P = 0.030
mic < mas P = 0.003
|
|
sic
|
20.55
|
3.07
|
15
|
27
|
|
mic
|
17.86
|
3.26
|
8
|
25
|
|
tyr
|
21.60
|
4.99
|
13
|
39
|
|
mas
|
23.55
|
2.30
|
15
|
30
|
|
18. Outermost involucral bract length (mm)2
|
ita
|
1.53
|
0.29
|
1
|
3
|
0.000
|
mas > ita P = 0.000
mas > sic P = 0.000
mas > mic P = 0.033
mas > tyr P = 0.007
sic < mic P = 0.006
sic < tyr P = 0.001
|
|
sic
|
1.21
|
0.14
|
1
|
1.9
|
|
mic
|
1.66
|
0.26
|
0.7
|
2.4
|
|
tyr
|
1.67
|
0.24
|
1
|
2.5
|
|
mas
|
1.95
|
0.18
|
1.2
|
2.5
|
|
19. Outermost involucral bract width (mm)2
|
ita
|
0.86
|
0.18
|
0.4
|
1.5
|
>0.05
|
|
|
sic
|
0.78
|
0.15
|
0.4
|
1.4
|
|
mic
|
0.73
|
0.08
|
0.4
|
1.2
|
|
tyr
|
0.86
|
0.14
|
0.4
|
1.8
|
|
mas
|
0.83
|
0.10
|
0.5
|
1.2
|
|
20. Outermost involucral bract length / outermost involucral bract width2
|
ita
|
1.87
|
0.49
|
1.07
|
4
|
0.000
|
mic > ita P = 0.020
mic > sic P = 0.038
mas > ita P = 0.002
mas > sic P = 0.012
|
|
sic
|
1.64
|
0.32
|
1
|
3
|
|
mic
|
2.34
|
0.36
|
1.17
|
4
|
|
tyr
|
2.07
|
0.55
|
0.92
|
4.6
|
|
mas
|
2.42
|
0.36
|
1.4
|
4
|
|
21. Outermost involucral bract texture. 0: bract totally papery; 0.5: bract herbaceous in its basal half and papery in its distal half; 1: bract totally herbaceous1
|
ita
|
0.67
|
0.29
|
0.5
|
1
|
0.048
|
|
|
sic
|
0.50
|
0
|
0.5
|
0.5
|
|
mic
|
0.75
|
0.25
|
0.5
|
1
|
|
tyr
|
0.65
|
0.25
|
0
|
1
|
|
mas
|
0.83
|
0.24
|
0.5
|
1
|
|
22. Eglandular indumentum of outermost involucral bract. 0: 0–5% coverage; 1: 6–25% coverage; 2: 26–50% coverage; 3: 51–75% coverage; 4: 76–100% coverage1
|
ita
|
3.34
|
0.78
|
2
|
4
|
>0.05
|
tyr > ita P = 0.043
|
|
sic
|
3.66
|
0.51
|
3
|
4
|
|
mic
|
3.71
|
0.46
|
3
|
4
|
|
tyr
|
3.71
|
0.45
|
3
|
4
|
|
mas
|
3.66
|
0.48
|
3
|
4
|
|
23. Glandular indumentum of outermost involucral bract. 0: 0–5% coverage; 1: 6–25% coverage; 2: 26–50% coverage; 3: 51–75% coverage; 4: 76–100% coverage2
|
ita
|
1.07
|
0.85
|
0
|
2.5
|
0.040
|
mas > ita P = 0.029
|
|
sic
|
1.50
|
0.63
|
1
|
2.5
|
|
mic
|
1.14
|
0.71
|
0
|
2
|
|
tyr
|
1.36
|
0.67
|
0
|
3
|
|
mas
|
1.73
|
0.53
|
0.5
|
2.5
|
|
24. Innermost involucral bract length (mm)3
|
ita
|
4.13
|
0.40
|
3.2
|
5.5
|
0.000
|
mas > ita P = 0.000
mas > mic P = 0.003
mas > tyr P = 0.000
|
|
sic
|
4.31
|
0.55
|
3
|
5
|
|
mic
|
4.10
|
0.32
|
3.4
|
5
|
|
tyr
|
4.03
|
0.35
|
2.5
|
5
|
|
mas
|
4.65
|
0.32
|
3.5
|
5.4
|
|
25. Innermost involucral bract width (mm)2
|
ita
|
0.88
|
0.06
|
0.5
|
1.2
|
0.004
|
sic > ita P = 0.004
sic > mic P = 0.012
sic > tyr P = 0.011
mas > ita P = 0.009
mas > tyr P = 0.033
|
|
sic
|
1.05
|
0.10
|
0.8
|
1.4
|
|
mic
|
0.88
|
0.09
|
0.5
|
1.3
|
|
tyr
|
0.90
|
0.13
|
0.3
|
1.3
|
|
mas
|
0.99
|
0.07
|
0.5
|
1.4
|
|
26. Innermost involucral bract length / innermost involucral bract width2
|
ita
|
4.77
|
0.63
|
2.92
|
8.2
|
>0.05
|
|
|
sic
|
4.14
|
0.22
|
3.5
|
5.44
|
|
mic
|
4.78
|
0.74
|
3.33
|
8.33
|
|
tyr
|
4.73
|
1.12
|
2.91
|
13.33
|
|
mas
|
4.80
|
0.40
|
2.86
|
9
|
|
27. Eglandular indumentum of innermost involucral bract. 0: 0–5% coverage; 1: 6–25% coverage; 2: 26–50% coverage; 3: 51–75% coverage; 4: 76–100% coverage1
|
ita
|
1.36
|
0.94
|
0
|
3
|
0.000
|
sic > ita P = 0.006
sic > mic P = 0.000
sic > tyr P = 0.000
sic > mas P = 0.000
mic < ita P = 0.001
mic < tyr P = 0.015
mas < ita P = 0.001
mas < tyr P = 0.020
|
|
sic
|
2.58
|
0.49
|
2
|
3
|
|
mic
|
0.35
|
0.41
|
0
|
1
|
|
tyr
|
1.11
|
0.81
|
0
|
3
|
|
mas
|
0.40
|
0.47
|
0
|
1
|
|
28. Glandular indumentum of innermost involucral bract. 0: 0–5% coverage; 1: 6–25% coverage; 2: 26–50% coverage; 3: 51–75% coverage; 4: 76–100% coverage1
|
ita
|
2.19
|
0.51
|
1
|
3
|
>0.05
|
|
|
sic
|
1.91
|
0.37
|
1.5
|
2.5
|
|
mic
|
2.14
|
0.30
|
2
|
3
|
|
tyr
|
2.31
|
0.52
|
1.5
|
3
|
|
mas
|
2.10
|
0.63
|
1
|
3
|
|
29. Average of innermost involucral bract length / outermost involucral bract length2
|
ita
|
2.75
|
0.43
|
1.83
|
3.64
|
0.000
|
sic > ita P = 0.000
sic > mic P = 0.000
sic > tyr P = 0.000
sic > mas P = 0.000
ita > tyr P = 0.002
ita > mas P = 0.022
|
|
sic
|
3.57
|
0.40
|
3.17
|
4.1
|
|
mic
|
2.50
|
0.36
|
1.86
|
2.97
|
|
tyr
|
2.45
|
0.37
|
1.58
|
3.59
|
|
mas
|
2.39
|
0.18
|
2.07
|
2.88
|
|
30. Number of involucral bracts per capitulum2
|
ita
|
29.44
|
3,20
|
22
|
40
|
0.012
|
mas > mic P = 0.044
mas > tyr P = 0.049
|
|
sic
|
30.38
|
1,10
|
26
|
36
|
|
mic
|
26.90
|
3,63
|
20
|
39
|
|
tyr
|
27.89
|
3,97
|
18
|
40
|
|
mas
|
30.75
|
4,03
|
19
|
38
|
|
31. Hermaphroditic florets length2
|
ita
|
3.55
|
0.34
|
2.7
|
4.4
|
>0.05
|
|
|
sic
|
3.58
|
0.48
|
3
|
4.4
|
|
mic
|
3.28
|
0.33
|
2.7
|
4
|
|
tyr
|
3.44
|
0.4
|
2.5
|
4.4
|
|
mas
|
3.74
|
0.41
|
2.3
|
4.3
|
|
32. Pistillate florets length2
|
ita
|
3.14
|
0.33
|
2.3
|
4
|
0.031
|
tyr < mas P = 0.037
|
|
sic
|
3.18
|
0.49
|
2.5
|
4.1
|
|
mic
|
2.95
|
0.31
|
2.3
|
3.5
|
|
tyr
|
3.05
|
0.38
|
2
|
4
|
|
mas
|
3.3
|
0.32
|
2.4
|
4
|
|
33. Pappus setae length2
|
ita
|
3.16
|
0.39
|
2.2
|
4
|
0.001
|
mas > ita P = 0.001
mas > micro P = 0.028
mas > tyr P = 0.012
|
|
sic
|
3.44
|
0.56
|
2.5
|
4.3
|
|
mic
|
3.05
|
0.31
|
2.2
|
3.9
|
|
tyr
|
3.13
|
0.39
|
2.1
|
4.1
|
|
mas
|
3.54
|
0.39
|
2.6
|
4.2
|
1 Kruskal-Wallis and Bonferroni as post-hoc test.
2 ANOVA and Tukey as post-hoc test.
3 Previously transformation to log, ANOVA and Tukey as post-hoc test. |
|