ARTÍCULO
Are plant conservation and war compatible?
The role of areas under dispute, military areas and military relics as nature reserves
S. MASSÓ1,2, C. BLANCHÉ2, L. SÁEZ3,4 & J. LÓPEZ-PUJOL1
1 BioC-GReB, Botanic Institute of Barcelona (IBB, CSIC-ICUB), pg. del Migdia, s/n, ES-08038 Barcelona, Catalonia, Spain
2 BioC-GReB, Laboratori de Botànica, Facultat de Farmàcia i Ciències de l’Alimentació, Universitat de Barcelona, av. Joan XXIII, s/n, ES-08028 Barcelona, Catalonia, Spain
3 Unitat de Botànica, Facultat de Biociències, Universitat Autònoma de Barcelona, ES-08193 Barcelona, Catalonia, Spain
4 Societat d’Història Natural de les Illes Balears (SHNB), c. Margarida Xirgu, 16, ES-07003 Palma de Mallorca, Balearic Islands, Spain
ORCID ID. S. MASSÓ: http://orcid.org/0000-0003-3786-4331, C. BLANCHÉ: http://orcid.org/0000-0001-8112-4635,
L. SÁEZ: http://orcid.org/0000-0003-4551-2432, J. LÓPEZ-PUJOL: http://orcid.org/0000-0002-2091-6222.
Author for correspondence: J. López-Pujol (jlopez@ibb.csic.es)
Editor: N. Ibáñez
Are plant conservation and war compatible? The role of areas under dispute, military areas and military relics as nature reserves
ABSTRACT
Are plant conservation and war compatible? The role of areas under dispute, military areas and military relics as nature reserves.— Wars and military activities have severe impacts on humans and on biodiversity, which are briefly summarized. Some side effects, although not ethically acceptable as principles, produced, however, some opportunities that have ultimately resulted in actions beneficial for plant conservation. A short review of case studies from all over the world and historical periods shows how military zones and activities can be turned on nature reserves if appropriate administrative decisions (scientifically based) are taken in the wider framework of concerted conservation with other areas of human intervention on the biosphere..
KEY WORDS: conservation; military; nature reserves; plant diversity; war.
¿Son compatibles la conservación de plantas y las guerras? El papel de las áreas en disputa, las áreas militares y las reliquias militares como reservas naturales
RESUMEN
¿Son compatibles la conservación de plantas y las guerras? El papel de las áreas en disputa, las áreas militares y las reliquias militares como reservas naturales.— Las guerras y las actividades militares en general tienen un gran impacto en los humanos y en la biodiversidad, que se resume aquí brevemente. Algunos efectos adversos, aunque no éticamente aceptables en principio, producen sin embargo algunas oportunidades que, en última instancia, dieron lugar a acciones beneficiosas para la conservación de las plantas. Una breve revisión de diferentes casos de estudio alrededor del mundo y en diferentes períodos históricos muestran cómo las zonas y actividades militares pueden actuar de reservas naturales si se toman las decisiones administrativas apropiadas (con base científica) en un marco más amplio de conservación coordinada con otras áreas de la intervención humana en la biosfera.
PALABRAS CLAVE: conservación; diversidad vegetal; guerra; militar; reservas naturales.
Recibido: 27/11/2017 / Aceptado: 03/04/2018 / Publicado on line: 08/01/2019
Cómo citar este artículo / Citation: Massó, S., Blanché, C., Sáez, L. & López-Pujol, J. 2018. Are plant conservation and war compatible? The role of areas under dispute, military areas and military relics as nature reserves. Collectanea Botanica 37: e009. https://doi.org/10.3989/collectbot.2018.v37.009
Copyright: © 2018 CSIC. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License.
CONTENIDOS
IMPACTS OF WAR ON PLANT DIVERSITYTop
The impacts of war on plants can be traced back to the earliest wars and military conflicts. One of the first examples is the purportedly intentional devastation of Greek olive trees [except those sacred individuals from the Delphi sanctuary area, once considered the center of the world—Omphalos—and today UNESCO World Heritage Site (UNESCO, 1992–2016UNESCO 1992–2016. World Heritage List. Archeological site of Delphi. UNESCO World Heritage Centre, Paris. Retrieved July 2, 2016, from http://whc.unesco.org/en/list/393)] during Peloponnesian War (5th century BC). Historically, the use of deliberate fires and other means of agricultural devastation to destroy forests and the natural resources stocks of the enemy has been a military well-known strategy (Rackman, 1990Rackman, O. 1990. Ancient landscapes. In: Murray, O. & Price, S. (Eds.), The Greek city. From Homer to Alexander. Oxford University Press Oxford: 85–111.; Davis, 1998Davis, V. H. 1998. Warfare and agriculture in Classical Greece (revised ed.). University of California Press, Berkeley & Los Angeles. ).
Significant impacts leading to forest destruction and subsequent erosion and soil loss have been also reported from classical times, to obtain materials for armies’ equipment and building, e.g. woodcutting for supplying construction of military buildings (forts, bridges) and especially shipbuilding. A documented massive habitat transformation in Roman times is reported from the Campania region at the area of Puteoli-Baiae (today Puzzoli, Italy). In 37 BC, Portus Julius—a military harbor—was built on the seashore next to Lake Lucrinus, a military shipyard installed at the base of a narrow crater, and a canal was dug to connect Lake Avernus to Lake Lucrinus. Up to 20,000 workers were employed in this extraordinary undertaking which resulted in an ecological catastrophe that was confirmed in modern times by the study of plant remains preserved in the lake’s sediments (Grüger et al., 2002Grüger, E., Thulin, B., Müller, J., Schneider, J., Alefs, J. & Welter-Schultes, F. W. 2002. Environmental changes in and around Lake Avernus in Greek and Roman times: A study of the plant and animal remains preserved in the lake’s sediments. In: Jashemski, W. F. & Meyer, F. G. (Eds.), The natural history of Pompeii. Cambridge University Press, Cambridge: 240–273.). Also direct weapons production implied natural resources extraction (such as massive Taxus baccata L. exploitation during Middle Age conflicts to produce thousands of bows implying cutting of thousands of yew tree branches, especially in Spain; cf. Pastoreau, 2004Pastoreau, M. 2004. Une histoire symbolique du Moyen Âge occidental. Éditions du Seuil, Paris. ; Mortimer, 2009Mortimer, I. 2009. 1415: Henry V’s year of glory. Random House, London.; Loades, 2013Loades, M. 2013. The longbow. Osprey Publishing, Oxford.).
New consequences of military campaigns are characteristic of the 16–18th centuries during the so-called “discovery expeditions”. In fact, together with the sending of naturalists to explore and describe diversity and uses of plant species of the newly acquired colonies (see for instance the Ruiz & Pavón expedition to Peru; Steele, 1982Steele, A. R. 1982. Flores para el rey. Ediciones del Serbal, Madrid. ), imperial expansions resulted in severe threats to the biodiversity of the “discovered” territories. The mixed commercial/military objectives (or trade goals with military tools) for the conquerors’ armies makes difficult to clearly segregate causes and consequences of such expeditions. In the Americas, for example, the Spanish, Portuguese or English colonists (1) cleared, burned and destroyed forests; (2) together with human infectious diseases, introduced plant seeds (some Old World crops but also plant invaders were released at that time), and (3) perturbed traditional knowledge and use of plant diversity (Crosby, 1988Crosby, A. W. 1988. Imperialismo ecológico: la expansión biológica de Europa, 900-1900. Editorial Crítica, Barcelona. ). Those activities were designed, controlled and/or implemented by the imperial armies. Some of these facts were even denounced by some of the contemporary participants, as the Spanish Dominican friar Fray Bartolomé de las Casas (1484–1566) who wrote “A short account of the destruction of the Indies” (Brevísima relación de la destrucción de las Indias; cf. Perales & Aguirre, 2008Perales, H. R. & Aguirre, J. R. 2008. Biodiversidad humanizada. In: Soberón, J., Halffter, G. & Llorente-Bousquets, J. (Coords.), Capital natural de México 1: Conocimiento actual de la biodiversidad. CONABIO, Ciudad de México: 565–603. ; Mira, 2009Mira, E. 2009. Conquista y destrucción de las Indias. Muñoz Moya Editor, Sevilla. ).
Perhaps one of the most outstanding examples of such combined military/commercial character of colonization times can be found in the activities of the Dutch East Indian Company (Vereenigde Oost-Indische Compagnie, VOC) in charge of the monopoly on Dutch spice trade. The company was set up in 1602 to profit from the Moluccan spice trade operating in the Indonesian seas (following Portuguese and preceding British dominations). The VOC has been considered the first multinational corporation in the world, acting during two centuries; together with political and trade missions, however, the company also exercised military powers through the own VOC army, including soldiers, guns, forts and fortresses or war ships (Ricklefs, 1991Ricklefs, M. C. 1991. A history of modern Indonesia since c. 1300 (2nd ed.). Macmillan, London.). In the 1620s, almost the entire native population of the Banda Islands was driven away, starved to death or killed in an attempt to replace them with Dutch plantations (Ricklefs, 1991Ricklefs, M. C. 1991. A history of modern Indonesia since c. 1300 (2nd ed.). Macmillan, London.). VOC representatives sometimes used the tactic of burning spice trees to force indigenous populations to grow other crops, thus artificially cutting the supply of spices like nutmeg (Myristica fragrans Houtt.) and cloves [Syzygium aromaticum (L.) Merr. & L. M. Perry]. Deliberate elimination of wild nutmeg trees in wide areas to concentrate the only remaining individuals in a few islands to control production and increase prices is a military action clearly oriented to biodiversity destruction (Hanna, 1978Hanna, W. A. 1978. Indonesian Banda: Colonialism and its aftermath in the Nutmeg Islands. Institute for the Study of Human Issues, Philadelphia.; Miller, 1996Miller, G. (Ed.) 1996. To the spice islands and beyond: travels in eastern Indonesia. Oxford University Press, New York.; Ames, 2008Ames, G. J. 2008. The globe encompassed: the age of European discovery, 1500–1700. Prentice Hall, London.) and early reported (e.g. by Rudloff, 1791Rudloff, F. V. 1791 The natural history of the nutmeg. Edinburgh Magazine 13: 129–131.). Fortunately, the programme was partially sabotaged by fruit pigeons that swallowed whole seeds and dispersed them to neighboring islands (including Run, a British possession, then breaking the forced isolation; cf. Weiss, 2002Weiss, E. A. 2002. Spice crops. CABI Publishing, London & New York. https://doi.org/10.1079/9780851996059.0000).
Since then, multiple effects of wars and armies on biodiversity have been repeatedly reported up to the contemporary armed conflicts. The World War II is plenty of examples of the negative impacts of war on plant conservation, a logical consequence of the significant increase in destructive capacities of weapons compared to former conflicts. It is well known the destruction of a great part of Berlin Herbarium—one of the largest in the world in that time—in an Allied bombing raid on March of 1943 (Fig. 1A). Elmer D. Merrill wrote in Science in December of 1943: “The loss of the Berlin herbarium is a catastrophe of major proportions to world botany” (Merrill, 1943Merrill, E. D. 1943. Destruction of the Berlin Herbarium. Science 98: 490–491. https://doi.org/10.1126/science.98.2553.490). The siege of Leningrad by the Wehrmacht (September 1941–January 1944) was even more destructive for the Komarov Botanical Institute; losses included not only plants (all but one of its 25 greenhouses were destroyed by bombs, with the subsequent loss of most of the tropical and subtropical collections) but also human lives; a great part of the staff did not survive the war, either having died of starvation or having been killed in action (Shetler, 1967Shetler, S. G. 1967. The Komarov Botanical Institute: 250 years of Russian research. Smithsonian Institutional Press, Washington. https://doi.org/10.5962/bhl.title.46347; Fig. 1B). The N. I. Vavilov germplasm collection of the Institute of Plant Industry, also located in Leningrad and the world’s largest at that time, mostly survived intact thanks to the heroic efforts of the scientific and technical personnel (despite this heroism cost the lives of many of them; Loskutov, 1999Loskutov, I. G. 1999. Vavilov and his institute. A history of the world col1ection of plant genetic resources in Russia. International Plant Genetic Resources Institute, Rome.). In the Asian-Pacific theater, less well known but major losses in terms of plant conservation also existed. These included the Japanese bombing of Tianmu Shan Nature Reserve of eastern China (home of one of the claimed “wild” populations of Ginkgo biloba L. as well as other relict plant species; Del Tredici et al., 1992Del Tredici, P., Ling, H. & Yang, G. 1992. The Ginkgos of Tian Mu Shan. Conservation Biology 6: 202–209. https://doi.org/10.1046/j.1523-1739.1992.620202.x); the destruction of the Bureau of Science Herbarium of Manila in February of 1945 during the liberation of the city (Howard, 2000Howard, R. A. 2000. The role of botanists during World War II in the Pacific Theatre. In: MacLeod, R. (Ed.), Science and the Pacific War – Science and survival in the Pacific, 1939–1945. Kluwer Academic Publishers, Dordrecht: 83–118. https://doi.org/10.1007/978-94-010-9766-6_6); or the American bombing of Shinjuku Imperial Botanical Garden of Tokyo in May of 1945. It is estimated that more than one-half of Tokyo’s urban trees were burned by fire bombings during the period 1942–1945 (Cheng & McBride, 2006Cheng, S. & McBride, J. R. 2006. Restoration of the urban forests of Tokyo and Hiroshima following World War II. Urban Forestry & Urban Greening 5: 155–168. https://doi.org/10.1016/j.ufug.2006.07.003). In addition to the damage inflicted by bombs and other weapons, construction of World War II large military structures also impacted negatively on plant biodiversity; for example, it is not hard to imagine the enormous impacts that the construction of the mammoth “Atlantic Wall” (see below, section “Military relics”) would have had on coastal vegetation or the deforestation resulting from the building of dozens of airfields in Pacific islets and atolls.
|
Figure 1. (A), ruins of the Botanical Garden and Botanical Museum of Berlin-Dahlem after the 1943 air raid [left, herbarium and library buildings (photograph: ©Archives of the Botanic Garden and Botanical Museum Berlin); right, ruins of the Grosses Tropenhaus (Large Tropical Greenhouse), Berlin Botanical Garden (photograph taken in 1947 by Roman Vishniac, © Mara Vishniac Kohn, courtesy International Center of Photography)]; (B) first page of volume 12 of Flora of USSR. The main contributor of this volume, N. F. Goncharov, despite weakened by starvation during the winter 1941–1942 (as consequence of the Leningrad Blockade), managed to complete the taxonomic treatment of genus Astragalus and defend his thesis (which was focused on the genus); regrettably, he died of hunger shortly after, in February of 1942 (Shetler, 1967Shetler, S. G. 1967. The Komarov Botanical Institute: 250 years of Russian research. Smithsonian Institutional Press, Washington. https://doi.org/10.5962/bhl.title.46347).

[View full size] [Descargar tamaño completo] |
|
The Second Indochina War (the commonly known “Vietnam War”, 1955–1975) implied the most devastating effects on forests; the widespread use of defoliant agents such as Agent Orange and other herbicides is thought to have defoliated 14% of Vietnam’s forest cover and over 50% of its coastal mangroves (Hanson et al., 2009Hanson, T., Brooks, T. M. & Da Fonseca, G. A. B. et al. 2009. Warfare in biodiversity hotspots. Conservation Biology 23: 578–587. https://doi.org/10.1111/j.1523-1739.2009.01166.x). Probably the birth of the term “ecocide” to name the deliberate destruction of the environment as a military strategy (DeWeerdt, 2008DeWeerdt, S. 2008. War and the environment. World Watch Magazine 21(1): 14–21.) emerged at that time [although, as early seen, it is in fact a very old practice: the Bible records in the Book of Judges (ca. 10th century BC) that King Abimelech salted the fields of Shechem (Judges 9:45, although there is no evidence that sufficient amounts of salt were used to render large tracts of land unusable) and that Samson burned the fields of the Philistines (Judges 15:5)].
Napalm, which was also used massively in Vietnam by the Americans, caused heavy deforestation in the mountainous cloud forests of El Salvador during its civil war of 1979–1992 (Wisner, 2001Wisner, B. 2001. Risk and the neoliberal state: Why post-Mitch lessons didn’t reduce El Salvador’s earthquake losses. Disasters 25(3): 251–268. https://doi.org/10.1111/1467-7717.00176). Also in America, the Colombian conflict (1964–2016) meant the conversion of forests to cattle pastures and coca (Erythroxylum sp.) plantations following land occupation by guerrillas and paramilitaries (Álvarez, 2003Álvarez, M. D. 2003. Forests in the time of violence: conservation implications of the Colombian War. Journal of Sustainable Forestry 16: 49–70. https://doi.org/10.1300/J091v16n03_03). The Gulf War (1990–1991) inflicted a wound not only on the Iraqi-occupied Kuwait (e.g. the Iraqi invaders confiscated the Kuwait Herbarium, the associated library and other scientific collections; Heywood, 2013Heywood, V. H. 2013. Award of OPTIMA gold medal to professor Loutfy Boulos Tawadros. Laudatio by professor Vernon Heywood. Organization for the Phyto-Taxonomic Investigation of the Mediterranean Area (OPTIMA), Palermo. Retrieved July 4, 2016, from http://www.optima-bot.org/awards/boulos.htm; Determann, 2015Determann, J. M. 2015. Researching biology and evolution in the Gulf states – Networks of science in the Middle East. I. B. Tauris, London & New York.) but also on the whole region. The massive oil spill that followed the Iraqi sabotage of ca. 800 oil wells (over 1 million m3 of oil was released into the Persian Gulf) produced varied effects, including immediate changes in growth and reproduction due to the decreased sunlight produced by the smoke from the oil fires (Omar et al., 2009Omar, S. A. S., Bhat, N. R. & Asem, A. 2009. Critical assessment of the environmental consequences of the invasion of Kuwait, the Gulf War, and the aftermath. In: Kassim, T. A. & Barceló, D. (Eds.), Environmental consequences of war and aftermath. Springer, Berlin & Heidelberg: 141–170. https://doi.org/10.1007/978-3-540-87963-3_5) and the direct destruction of the vegetation cover (such as saltmarshes and mangroves; Böer, 1993Böer, B. 1993. Anomalous pneumatophores and adventitious roots of Avicennia marina (Forssk.) Vierh. mangroves two years after the 1991 Gulf War oil spill in Saudi Arabia. Marine Pollution Bulletin 27: 207–211. https://doi.org/10.1016/0025-326X(93)90026-G). The off-road movement of military vehicles and the construction of trenches and placement of mines also increased soil erosion and dune formation (see Sadiq & McCain, 1993Sadiq, M. & McCain, J. C. 1993. The Gulf War aftermath – An environmental tragedy. Kluwer Academic Publishers, Dordrecht. https://doi.org/10.1007/978-94-011-1685-5 and Omar et al., 2009Omar, S. A. S., Bhat, N. R. & Asem, A. 2009. Critical assessment of the environmental consequences of the invasion of Kuwait, the Gulf War, and the aftermath. In: Kassim, T. A. & Barceló, D. (Eds.), Environmental consequences of war and aftermath. Springer, Berlin & Heidelberg: 141–170. https://doi.org/10.1007/978-3-540-87963-3_5, for detailed information on the effects of the Gulf War).
In Africa, the Rwandan Civil War (1990–1994) had large and varied (although difficult to evaluate) impacts on plant diversity. In addition to the direct effects on plant species and their habitats (direct destruction of habitats by bombing, habitat fragmentation, erosion), all the research conservation activities were almost totally suppressed (Kanyamibwa, 1998Kanyamibwa, S. 1998. Impact of war on conservation: Rwandan environment and wildlife in agony. Biodiversity and Conservation 7: 1399–1406. https://doi.org/10.1023/A:1008880113990): most reserves were directly closed or their activities significantly diminished (as most of the their guards, researchers and conservationists either fled the country or were killed), whereas others lost large parts of their original size (e.g. Akagera National Park almost lost two-thirds of its original size) or were partly transformed into cutting and grazing areas. Moreover, collateral effects of this war also were lethal to the region’s plant diversity: the nearly million Rwandan refugees living around Virunga National Park in the neighboring Democratic Republic of Congo deforested about 300 km2 of the park to get firewood (McNeely, 2003McNeely, J. A. 2003. Conserving forest biodiversity in times of violent conflict. Oryx 37: 142–152. https://doi.org/10.1017/S0030605303000334); this tragedy, regrettably, should be added to the assassination of at least 80 Virunga’s park staff during the civil war in the Democratic Republic of Congo (1996–2003; McNeely, 2003McNeely, J. A. 2003. Conserving forest biodiversity in times of violent conflict. Oryx 37: 142–152. https://doi.org/10.1017/S0030605303000334).
The most recent war in Europe (the Yugoslav War, 1991–2001) also had enormous losses regarding plant conservation; during the terrific siege of Sarajevo, which lasted for almost four years (April 1992–March 1996), over three-quarters of all urban trees and nearly all peri-urban trees were cut down for firewood (Lacan & McBride, 2009Lacan, I. & McBride, J. R. 2009. War and trees: The destruction and replanting of the urban and peri-urban forest of Sarajevo, Bosnia and Herzegovina. Urban Forestry & Urban Greening 8: 133–148. https://doi.org/10.1016/j.ufug.2009.04.001), whereas many wild plant species were massively harvested to be used as food by hungry residents (Redžić, 2010Redžić, S. 2010. Use of wild and semi-wild edible plants in nutrition and survival of people in 1430 days of siege of Sarajevo during the war in Bosnia and Herzegovina (1992–1995). Collegium Antropologicum 34: 551–570.). The living collections of the Botanic Garden of Sarajevo were almost completely destroyed during the siege, including the valuable collections of Bosnian endemic and rare plants (BGCI, 1996BGCI [Botanic Gardens Conservation International] 1996. Proposals for the Reconstruction of the Zemaljski Museum and the Botanic Garden, Sarajevo. BGCI News 2(6). Retrieved July 3, 2016, from http://www.bgci.org/worldwide/article/0166/). In another Bosnian city that was almost reduced to rubble, Mostar, expansion of invasive species into the newly created habitats (such as ruins and burned areas) has been reported (Maslo, 2014Maslo, S. 2014. The urban flora of the city of Mostar (Bosnia and Herzegovina). Natura Croatica 23: 101–145.). Also within the European continent there is an example of a recent conflict that is still going on, the War in Eastern Ukraine (2014–present). This war has affected significant percentages of both forests and steppes within the region—including those located on nature reserves, by means of direct effects (explosions and fires, and damage due to the passage of heavy military machinery and construction of fortifications and trenches) or indirectly by dismantling nature reserves administration (Vasyliuk et al., 2017Vasyliuk, O., Shyriaieva, D., Kolomytsev, G. & Spinova, J. 2017. Steppe protected areas on the territory of Ukraine in the context of the armed conflict in the Donbas region and Russian annexation of the Crimean Peninsula. Bulletin of the Eurasian Dry Grassland Group 33: 15–23. https://doi.org/10.21570/EDGG.Bull.33.15-23). A number of additional cases can be found in the Enzler (2006Enzler, S. M. 2006. Environmental effects of warfare. Lenntech BV Delft. Retrieved July 3, 2016, from http://www.lenntech.com/environmental-effects-war.htm) webpage about the environmental effects of wars and incidents leading to war that have occurred in the 20th and 21st century. As in many other fields of environmental conflicts, conservation problems are often not true biological problems in the origin, but socio-economic problems with biological consequences (see Folch, 2011Folch, R. 2011. La quimera de créixer. La sostenibilitat en l’era postindustrial. La Magrana, Barcelona.). Warfare is undoubtedly a good example.
CONVERSION OF MILITARY IMPACTS TO PLANT CONSERVATION BENEFITSTop
Some side effects, obviously not ethically acceptable as main drivers of any military activity, however, produced, by indirect ways or by the tenacity of botanists or plant-lovers, some kind of opportunities to increase plant knowledge during war conflicts or even to incredibly result in actions beneficial for plant conservation. Only in this sense, we could consider that war has not always had negative effects on plant conservation or that war consequences can be turned on conservation tools. There are many examples of opportunity effects of war (in any of its manifestations) through space and time, and these have covered several aspects of conservation including both basic knowledge and practical measures (see Table 1 in McNeely, 2003McNeely, J. A. 2003. Conserving forest biodiversity in times of violent conflict. Oryx 37: 142–152. https://doi.org/10.1017/S0030605303000334, for a summary). For example, botanists embedded in military expeditions include Theophrastus’ disciples in the army of Alexandre the Great (4th century BC; Amigues, 2010Amigues, S. 2010. Théophraste. Recherches sur les plantes. À l’origine de la botanique. Éditions Belin, Paris. ) or Pedanius Dioscorides (ca. 40–90 AD), who wrote his famous Περί ὕλης ἰατρικής (De Materia Medica in the Latin translation, a 5-volume pharmacopeia that was the standard reference on medicinal plants until the 17th century and that describes over 600 plant species) thanks to his job as a physician in the Roman army, and which gave him the opportunity to travel extensively through the Empire (Segura & Torres, 2009Segura, S. & Torres, J. 2009. Historia de las plantas en el mundo antiguo. CSIC-Universidad de Deusto, Bilbao & Madrid. ). More recently, the military campaigns of Napoleon Bonaparte in Egypt and Syria (1798–1801) allowed the botanical explorations of the French botanist Alire Raffeneau Delile (1778–1850), which constituted the basis for his Flore d’Égypte, published in 1813 (Solé, 1998Solé, R. 1998. Les savants de Bonaparte. Éditions du Seuil, Paris. ). Later on, the Opium Wars in Asia (middle 19th century) bolstered the plant exploration in China (Hu & Watson, 2015Hu, C.-M. & Watson, M. F. 2015. Plant exploration in China. In: Hong, D.-Y. & Blackmore, S. (Eds.), Plants of China – A companion to the Flora of China. Science Press, Beijing: 212–236.); in Hong Kong, which was ceded to Great Britain in 1842 after the Chinese defeat in the First Opium War (1839–1842), the first “complete” flora of any Chinese region was published in 1861 (the Flora Hongkongensis of George Bentham), whereas the first “modern” botanical garden of China (and, thus, the first ex situ conservation facility) was also established there in 1861 (López-Pujol et al., 2006López-Pujol, J., Zhang, F.-M. & Ge, S. 2006. Plant biodiversity in China: richly varied, endangered and in need of conservation. Biodiversity and Conservation 15: 3983–4026. https://doi.org/10.1007/s10531-005-3015-2). One of the few partly beneficial side effects of the civil war in the Democratic Republic of Congo (1996–2003) was the collapse of the wood industry. The country’s total timber production fell by about two-thirds, and, for the case of Équateur Province, it fell to zero (Draulans & Van Krunkelsven, 2002Draulans, D. & Van Krunkelsven, E. 2002. The impact of war on forest areas in the Democratic Republic of Congo. Oryx 36: 35–40. https://doi.org/10.1017/S0030605302000066). The possibility of a nuclear war is one of the main reasons behind the construction of the Global Seed Vault (also commonly known as “Doomsday Seed Vault”, officially opened in 2008) in the Norwegian Svalbard Archipelago (Charles, 2006Charles, D. 2006. A ‘Forever’ seed bank takes root in the Arctic. Science 312: 1730–1731. https://doi.org/10.1126/science.312.5781.1730b). By coincidence, the German soldiers stationed in the archipelago were the last military unit to surrender during the World War II, almost four months after the fall of Berlin.
One of the major opportunities to revert the undesirable effects of warfare is to, at least, take profit of the kidnapping of land extensions reserved for military activities, put then outside of the productive pressure leading to increasing habitats destruction or quality loss, one of the main drivers of the biodiversity crisis (Hassan et al., 2005Hassan, R., Scholes, R. & Ash, N. (Eds.) 2005. Ecosystems and human well-being: current state and trends 1. Island Press, Washington.). Such lands—sometimes of considerable surface—are, in general, of public (state) property and suitable for in situ conservation. The derived positive effects of war are, thus, mostly associated with the in situ conservation of plant species (sometimes referred as “gunpoint conservation”; Álvarez, 2003Álvarez, M. D. 2003. Forests in the time of violence: conservation implications of the Colombian War. Journal of Sustainable Forestry 16: 49–70. https://doi.org/10.1300/J091v16n03_03). There are many examples in which areas under dispute as well as military areas and facilities (including relics) have facilitated or at least enabled the conservation of plant species within their natural habitats, thus acting as de facto nature reserves. In a few cases, these areas have even reached legal status as protected areas (PAs). Although providing an exhaustive, systematic compilation of “military in situ plant conservation” examples throughout the world is beyond the scope of the present contribution, we are aimed to present some of the most representative and illustrative case-studies, which have been divided on three types based on conceptual and practical criteria: (1) areas under dispute, (2) military areas, and (3) military relics.
AREAS UNDER DISPUTETop
The Korean Demilitarized Zone (DMZ)
The Korean War (1950–1953) resulted in the permanent division of the Korean Peninsula into two politically-opposed countries: the Democratic People’s Republic of Korea in the north and the Republic of Korea in the south. Although there is no formal border between the two countries (given that there was no peace treaty following the ceasefire), the armistice involved the establishment of a Military Demarcation Line (MDL, the de facto border), which in fact does not run very far from the 38th parallel north—the original 1945 demarcation line between the United States and Soviet occupation zones (Fig. 2). The length of the MDL is about 248 km, and there is a buffer zone of ca. 2 km of width at each side of the MDL, called the Korean Demilitarized Zone (DMZ). The DMZ covers a total of 907 km2, and has remained a virtually “no-man’s land” for the last 65 years and, thus, a sanctuary for biodiversity (Kim, 1997Kim, K. C. 1997. Preserving biodiversity in Korea’s Demilitarized Zone. Science 278: 242–243. https://doi.org/10.1126/science.278.5336.242, 2013Kim, K.-G. 2013. The Demilitarized Zone (DMZ) of Korea – Protection, conservation and restoration of a unique ecosystem. Springer, Berlin & Heidelberg.). Moreover, along the southern boundary of the DMZ, the Civilian Controlled Zone (CCZ) was set for military purposes, with a width ranging from 5 to 20 km; the CCZ, with 1369 km2 (Kim, 2013Kim, K.-G. 2013. The Demilitarized Zone (DMZ) of Korea – Protection, conservation and restoration of a unique ecosystem. Springer, Berlin & Heidelberg.), has become an area where human activities are strictly limited (only some farming is allowed; Kim et al., 2011Kim, J.-O., Steiner, F. & Mueller, E. 2011. Cranes, crops and conservation: Understanding human perceptions of biodiversity conservation in South Korea’s Civilian Control Zone. Environmental Management 47: 1–10. https://doi.org/10.1007/s00267-010-9568-1). Altogether, both areas account for almost 2300 km2 of relatively pristine lands that are acting as a de facto nature reserve.
|
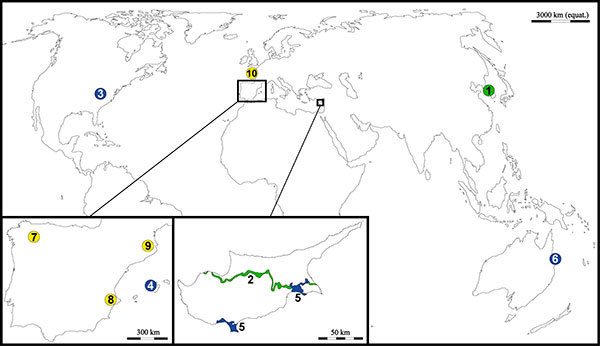
Figure 2. Geographical situation of the ten “military in situ plant conservation” examples. In green, areas under dispute; in blue, military areas; in yellow, military relics. (1), the Korean Demilitarized Zone (DMZ); (2), the Green Line (Cyprus); (3), Fort Bragg (North Carolina, USA); (4), Puig Major Air Force Radar Base (Spain); (5), Sovereign Base Areas of Akrotiri and Dhekelia (Cyprus); (6), Shoalwater Bay Military Training Area (Queensland, Australia); (7), Cornatel Castle (Spain); (8), Xàtiva Castle (Spain); (9), Sant Ferran Fortress (Spain); (10), Longues-sur-Mer Battery (and other sites related to 1944 Normandy landings) (France).
[View full size] [Descargar tamaño completo] |
|
Given that CCZ and, especially DMZ, have remained almost untouched since the 1950s, it is not surprising that this area may harbor ca. 1600 plant species (Kim et al., 2011Kim, J.-O., Steiner, F. & Mueller, E. 2011. Cranes, crops and conservation: Understanding human perceptions of biodiversity conservation in South Korea’s Civilian Control Zone. Environmental Management 47: 1–10. https://doi.org/10.1007/s00267-010-9568-1); that is, a remarkable one third of the total vascular flora estimated for the Korean Peninsula (4662 species; Kim, 2006Kim, Y.-S. 2006. Conservation of plant diversity in Korea. Landscape and Ecological Engineering 2: 163–170. https://doi.org/10.1007/s11355-006-0004-x) is found in a mere 1% of its total area. Figures are even more significant for animals: 71% of all amphibians and reptiles, 51% of all birds, and 52% of all mammals native to Korea are found in this narrow strip (Kim & Cho, 2005Kim, K.-G. & Cho, D.-G. 2005. Status and ecological resource value of the Republic of Korea’s De-militarized Zone. Landscape and Ecological Engineering 1: 3–15. https://doi.org/10.1007/s11355-005-0006-0; Kim et al., 2011Kim, J.-O., Steiner, F. & Mueller, E. 2011. Cranes, crops and conservation: Understanding human perceptions of biodiversity conservation in South Korea’s Civilian Control Zone. Environmental Management 47: 1–10. https://doi.org/10.1007/s00267-010-9568-1). According to Kim (1997Kim, K. C. 1997. Preserving biodiversity in Korea’s Demilitarized Zone. Science 278: 242–243. https://doi.org/10.1126/science.278.5336.242), the DMZ and the CCZ provide wintering habitats for two of the world’s most endangered birds, red-crowned crane [Grus japonensis (Statius Muller, 1776)] and white-naped crane (Grus vipio Pallas, 1811), and are also home of threatened, iconic animals such as the Asian black bear (Ursus thibetanus G.[Baron] Cuvier, 1823) and the Siberian musk deer (Moschus moschiferus Linnaeus, 1758). The DMZ plus the CCZ is also rich in ecosystem diversity despite the area was heavily farmed before the Korean War; many natural habitats have naturally recovered after more than half a century of abandonment (Kim, 2013Kim, K.-G. 2013. The Demilitarized Zone (DMZ) of Korea – Protection, conservation and restoration of a unique ecosystem. Springer, Berlin & Heidelberg.). At present, it is estimated that natural habitats represent about 97% of the DMZ and 85% of the CCZ (Kim, 2001Kim, K.-G. 2001. A study on the feasibility as well as an operational strategy to develop DMZ Transboundary Biosphere Reserve between DPR Korea and Republic of Korea – Final report. UNESCO Jakarta Office, Jakarta., 2013Kim, K.-G. 2013. The Demilitarized Zone (DMZ) of Korea – Protection, conservation and restoration of a unique ecosystem. Springer, Berlin & Heidelberg.). On the flattest part of the DMZ+CCZ (their western section), wetlands are extensive and varied, with part of them being derived from old rice paddy fields (Kim & Cho, 2005Kim, K.-G. & Cho, D.-G. 2005. Status and ecological resource value of the Republic of Korea’s De-militarized Zone. Landscape and Ecological Engineering 1: 3–15. https://doi.org/10.1007/s11355-005-0006-0; Kim, 2013Kim, K.-G. 2013. The Demilitarized Zone (DMZ) of Korea – Protection, conservation and restoration of a unique ecosystem. Springer, Berlin & Heidelberg.). The central section is a mix of forests, plains, and marshes, whereas the eastern section is formed by the rugged mountains of the Baekdudaegan—the longest mountain chain in NE Asia, stretching ca. 1625 km from Mt. Baekdu in North Korea to Mt. Jiri in South Korea, a regional major glacial refugium for plants (Chung et al., 2017Chung, M. Y., López-Pujol, J. &, Chung, M. G. 2017. The role of the Baekdudaegan (Korean Peninsula) as a major glacial refugium for plant species: A priority for conservation. Biological Conservation 206: 236–248. https://doi.org/10.1016/j.biocon.2016.11.040).
The DMZ+CCZ have been repeatedly suggested being a transboundary protected area (PA) (e.g. Kim, 1997Kim, K. C. 1997. Preserving biodiversity in Korea’s Demilitarized Zone. Science 278: 242–243. https://doi.org/10.1126/science.278.5336.242, 2001Kim, K.-G. 2001. A study on the feasibility as well as an operational strategy to develop DMZ Transboundary Biosphere Reserve between DPR Korea and Republic of Korea – Final report. UNESCO Jakarta Office, Jakarta., 2013Kim, K.-G. 2013. The Demilitarized Zone (DMZ) of Korea – Protection, conservation and restoration of a unique ecosystem. Springer, Berlin & Heidelberg.; Kim & Cho, 2005Kim, K.-G. & Cho, D.-G. 2005. Status and ecological resource value of the Republic of Korea’s De-militarized Zone. Landscape and Ecological Engineering 1: 3–15. https://doi.org/10.1007/s11355-005-0006-0; IUCN, 2014IUCN [International Union for Conservation of Nature] 2014. A statement on transboundary conservation for biodiversity and peace. IUCN, Gland. Retrieved March 2, 2016, from http://cmsdata.iucn.org/downloads/biodiversity_and_peace_statement_final_approved_at_cbd_event.pdf). Several aspects make this area as highly suitable to become a transboundary PA, including: (1) is a well-defined, perfectly controlled area; (2) the hypothetical PA could be even larger if the counterpart of the South Korean CCZ (which is known to exist; Kim 2001Kim, K.-G. 2001. A study on the feasibility as well as an operational strategy to develop DMZ Transboundary Biosphere Reserve between DPR Korea and Republic of Korea – Final report. UNESCO Jakarta Office, Jakarta.) is added; (3) the new PA will effectively link two of the most important PAs within the Korean Peninsula (Mount Geumgang in North Korea and Mount Seoraksan in South Korea), which are part of the Baekdudaegan, one of the major biological corridors of East Asia; one of the recommendations adopted at the IUCN 2012 World Conservation Congress (IUCN, 2012IUCN [International Union for Conservation of Nature] 2012. Resolutions and Recommendations. World Conservation Congress, Jeju, Republic of Korea, 6–15 September 2012. IUCN, Gland.) was the creation of the very ambitious “Ecological Corridor of Northeast Asia”, which would integrate the BDDG, Mt. Changbai in China, Tumen River basin, and the mountain system of Sikhote-Alin in Russian Far East; (4) in the South Korean side, several sites already constitute PAs (including a Ramsar site, Yongneup Moors; Kim, 2013Kim, K.-G. 2013. The Demilitarized Zone (DMZ) of Korea – Protection, conservation and restoration of a unique ecosystem. Springer, Berlin & Heidelberg.); and (5) setting such a transboundary PA, especially if constitutes a “Peace Park” or a similar figure (Sandwith et al., 2001Sandwith, T., Shine, C., Hamilton, L. & Sheppard, D. 2001. Transboundary protected areas for peace and co-operation. IUCN, Gland & Cambridge.; Ali, 2007Ali, S. H. (Ed.) 2007. Peace Parks: Conservation and conflict resolution. MIT Press, Cambridge.) could be a way to improve the political relationships between the two countries and to promote inter-Korean reconciliation.
Since the late 1990s, the South Korean government has repeatedly proposed the creation of a transboundary area in the DMZ, but the North Korean counterpart has always refused to participate in planning this concept (Kim, 1997Kim, K. C. 1997. Preserving biodiversity in Korea’s Demilitarized Zone. Science 278: 242–243. https://doi.org/10.1126/science.278.5336.242, 2013Kim, K.-G. 2013. The Demilitarized Zone (DMZ) of Korea – Protection, conservation and restoration of a unique ecosystem. Springer, Berlin & Heidelberg.; Hayes, 2010Hayes, P. 2010. Sustainable security in the Korean Peninsula: envisioning a northeast Asian biodiversity corridor. Korean Journal of International Studies 8: 197–230.). One of the last attempts was in early 2010s, when South Korean government tried to register the southern part of the DMZ (and a large part of the CCZ) area as an UNESCO Biosphere Reserve, with no success (Mok, 2012Mok, J.-M. 2012. UNESCO denies designation of DMZ as Biosphere Reserve: End of hasty efforts neglecting recommendations for North-South joint designation. Kyunghyang Shinmun, July 13, 2012. Retrieved July 6, 2016, from http://english.khan.co.kr/khanartview.html?artid=201207131046497&code=710100). The failure in protecting the DMZ+CCZ would lead to the increase of pressures on natural habitats in the short- and middle-term. For example, farming and other development pressures have increased in recent years (Kim et al., 2011Kim, J.-O., Steiner, F. & Mueller, E. 2011. Cranes, crops and conservation: Understanding human perceptions of biodiversity conservation in South Korea’s Civilian Control Zone. Environmental Management 47: 1–10. https://doi.org/10.1007/s00267-010-9568-1; Kim, 2013Kim, K.-G. 2013. The Demilitarized Zone (DMZ) of Korea – Protection, conservation and restoration of a unique ecosystem. Springer, Berlin & Heidelberg.), and these pressures may be even larger if the area of CCZ is reduced, as planned by the government (Yonhap, 2014Yonhap 2014. S. Korea considers reducing civilian control area near DMZ. Yonhap News Agency, May 7, 2014. Retrieved June 21, 2016, from http://english.yonhapnews.co.kr/full/2014/05/07/31/1200000000AEN20140507008700315F.html?76ea0780). The hypothetical reunification might also mean a severe fragmentation of the frontier region, especially if this is not protected; in a simulation under a scenario of reunification, Sung (2015Sung, C. Y. 2015. Simulation of crane habitat fragmentation in the North and South Korean border region after Korean reunification. Landscape and Urban Planning 134: 10–18. https://doi.org/10.1016/j.landurbplan.2014.10.008) reported that the habitats of Grus japonensis and G. vipio will be seriously fragmented even in the case of protection of the border area (roads and other infrastructures will be essential to reunify North and South Korea).
The Green Line (Cyprus)
The recent history of Cyprus (including its complicated process of independence from the British Empire), an island located in the Eastern Mediterranean Basin, has left two “military scars”: the Sovereign Base Areas of Akrotiri and Dhekelia (see their own case-study, below), which were retained by the British under the 1960 treaty of independence, and the United Nations Buffer Zone in Cyprus (also known as the “Green Line”), which is a demilitarized zone whose current extension was established in 1974 after the Turkish invasion of Cyprus (Fig. 2).
Since 1964, some areas of Cyprus were divided through ethnic lines between Greek and Turkish people. These two communities were confronted since the independence of the island in 1960, and intercommunal violence was common throughout the island. The current United Nations Buffer Zone in Cyprus was established in 1974 following a military coup d’état and the subsequent Turkish invasion of the island, and divides Cyprus in two regions. The southern area is controlled by the Republic of Cyprus whereas the northern third of the island is controlled by the self-proclaimed and internationally unrecognized Turkish Republic of Northern Cyprus. The Cyprus Buffer Zone stretches along 180 km, occupies ca. 3% of the island and acts as border between these two regions (Fig. 2). As for the case of the Korean Peninsula, the Green Line has remained quite undeveloped, which has greatly contributed to preserve its biodiversity (Grichting, 2014Grichting, A. 2014. Cyprus: Greening in the dead zone. In: Tidball, K. G. & Krasny, M. E. (Eds.), Greening in the red zone – Disaster, resilience and community greening. Springer, Dordrecht: 429–443. https://doi.org/10.1007/978-90-481-9947-1_33).
Cyprus is considered one of the ten biodiversity hotspots in the Mediterranean Basin (Médail & Quézel, 1999Médail, F. & Quézel, P. 1999. Biodiversity hotspots in the Mediterranean Basin: setting global conservation priorities. Conservation Biology 13: 1510–1513. https://doi.org/10.1046/j.1523-1739.1999.98467.x; Myers et al., 2000Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B. & Kent, J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. https://doi.org/10.1038/35002501). The varied microclimate and geology of the island, together with its isolation and location near large neighboring land masses, have greatly contributed to its rich biodiversity (Öztürk et al., 2011Öztürk, M., Gucel, S., Guvensen, A., Kadis, C. & Kounnamas, C. 2011. Halophyte plant diversity, coastal habitat types and their conservation status in Cyprus. In: Öztürk, M., Böer, B., Barth, H.-J., Breckle, S.-W., Clüsener-Godt, M. & Khan, M. A. (Eds.), Sabkha ecosystems 3: Africa and Southern Europe. Springer, Dordrecht: 99–111. https://doi.org/10.1007/978-90-481-9673-9) and 145 of the almost 2000 plant taxa of Cyprus are endemics (Tsintides et al., 2007Tsintides, T., Christodoulou, C. S., Delipetrou, P. & Georghiou, K. (Eds.) 2007. The red data book of the flora of Cyprus. Cyprus Forestry Association, Lefkosia.). At least two of these 145 Cypriot endemic species, Tulipa cypria Stapf ex Turrill and Ophrys kotschyi H. Fleischm. & Soó, grow in the Green Line together with other rare and endangered plants in Cyprus, e.g. Mandragora officinarum L. (Gücel et al., 2008Gücel, S., Charalambidou, I., Göçmen, B., Karataş, A., Özden, Ö., Soyumert, A. & Fuller, W. 2008. Monitoring biodiversity of the buffer zone in Cyprus. Poster presented at the “Monitoring biodiversity in Europe. Volunteers, efficiency and cost” congress (Leipzig, Germany, 28–30 January 2008).; Jarraud, 2008Jarraud, N. 2008. Hawks, doves and wild-sheep. Development and Transition 9(April): 9–11.). Following the Article 6 of the Habitats Directive, all European Union states should take the necessary measures in order to ensure the protection of species and habitats. Due to this, the United Nations Development Program (UNDP) and the European Commission funded a project between Greek- and Turkish-Cypriot scientists for conservation of endemic, rare and threatened plants. Two plant micro-reserves were created thanks to this project in Mammari and Denia villages, which are located within the Green Line. In Mammari, for example, the largest population of Ophrys kotschyi is located there and a new population of Tulipa cypria was observed in 2007 (Tsintides et al., 2007Tsintides, T., Christodoulou, C. S., Delipetrou, P. & Georghiou, K. (Eds.) 2007. The red data book of the flora of Cyprus. Cyprus Forestry Association, Lefkosia.) with a hundred of individuals (Trias-Blasi et al., 2017Trias-Blasi, A., Gücel, S. & Özden, Ö. 2017. Current distribution and conservation status reassessment of the Cyprus Tulip (Tulipa cypria: Liliaceae), new data from northern Cyprus. Plant Biosystems 151: 394–402. https://doi.org/10.1080/11263504.2016.1174177). Interestingly, the second largest population of Ophrys kotschyi is located in Akrotiri, one of the British bases (Tsintides et al., 2007Tsintides, T., Christodoulou, C. S., Delipetrou, P. & Georghiou, K. (Eds.) 2007. The red data book of the flora of Cyprus. Cyprus Forestry Association, Lefkosia.; see below).
MILITARY AREASTop
Fort Bragg (North Carolina, USA)
A relevant example of how military areas can be involved in biodiversity conservation is the integrated management plan for cultural and natural resources for Fort Bragg (North Carolina, USA; Fig. 2), a very important installation of the United States Army. Formerly known as Camp Bragg (named after confederate general Braxton Bragg), it was established in 1918 as an artillery training ground and today is one of the largest military bases in the world (by population) with more than 50,000 active duty personnel, including two airfields.
In the early 1990s, the newly established protection by the US Fish and Wildlife Service for the endangered red-cockaded woodpecker [Leuconotopicus borealis (Vieillot, 1809)] caused severe troubles for Fort Bragg including temporarily closing areas and moving of some installations. The conflict finally resulted in an agreement between the Army and conservationist groups (Gorsira et al., 1996Gorsira, B., Belfit, S. C. & Cantrell, M. A. 1996. Alleviating conflicts between army training and endangered species at Fort Bragg. Federal Facilities Environmental Journal 7: 59–67. https://doi.org/10.1002/ffej.3330070307), after which new conservation projects were progressively started in a win-win strategy.
In this framework, an integrated management plan for cultural and natural resources for Fort Bragg (Griffin et al., 2001Griffin, M., Boyko, W., Boyko, B. et al. 2001. Integrated cultural resources management plan (ICRMP) for Ft. Bragg, Camp Mackall, and Simmons Army Airfield. Final Draft. Griffin Social Technologies, Chesapeake.) arose without relevant adversities, thus assuring the conservation of several historical sites (there are nearly 5000 identified sites, including many native American archaeological remains; USAEC, 2007USAEC [US Army Environmental Command] 2007. Draft programmatic environmental impact statement for army growth and force structure realignment of the U.S. Army. US Department of the Army, Washington. ) coupled with several populations of federally endangered species (as Lysimachia asperulifolia Poir., Rhus michauxii Sarg., and Schwalbea americana L.), in an area of military ownership, previously explored and prioritized (Sorrie et al., 1997Sorrie, B. A., Van Eerden, B. & Russo, M. J. 1997. Noteworthy plants from Fort Bragg and Camp MacKall, North Carolina. Castanea 62: 239–259.).
Today, a well-established department of the military area is devoted to environmental issues (Fort Bragg DPW Environmental Division), including a specific conservation unit (Endangered Species Branch), although management also allows fishing and hunting activities (details in http://www.bragg.army.mil).
Puig Major Air Force Radar Base (Balearic Islands, Spain)
The Puig Major (1445 m a.s.l.) is the highest peak in Mallorca (Balearic Islands; Fig. 2). There is an extraordinary concentration of endemic plant species in this mountain (Sáez, 2010Sáez, L. 2010. Plantes endèmiques dels Països Catalans. In: Giralt, J., Comelles, M., Montagud, E. et al. (Eds.), Suplement Fauna i Flora. Història Natural dels Països Catalans. Enciclopèdia Catalana Barcelona: 161–164. ; López-Pujol et al., 2013López-Pujol, J., Martinell, M. C., Massó, S., Blanché, C. & Sáez, L. 2013. The ‘paradigm of extremes’: extremely low genetic diversity in anextremely narrow endemic species, Coristospermum huteri (Umbelliferae). Plant Systematics and Evolution 299: 439–446. https://doi.org/10.1007/s00606-012-0732-3; Galán de Mera & Sáez, 2016Galán de Mera, A. & Sáez, L. 2016. Taraxacum majoricense (Asteraceae), a new species from the Balearic Islands, Spain. Annales Botanici Fennici 53: 82–90. https://doi.org/10.5735/085.053.0216). The summit of Puig Major (which is a closed military zone) is a hotspot of Extremely Narrow Endemic (ENE) species and other endangered plants, since it has sufficient elevation to function in the present interglacial period as a refuge for several European montane species (López-Pujol et al., 2013López-Pujol, J., Martinell, M. C., Massó, S., Blanché, C. & Sáez, L. 2013. The ‘paradigm of extremes’: extremely low genetic diversity in anextremely narrow endemic species, Coristospermum huteri (Umbelliferae). Plant Systematics and Evolution 299: 439–446. https://doi.org/10.1007/s00606-012-0732-3).
Three war events in the history of the 20th century (Spanish Civil War, World War II and the Cold War) had a decisive impact on the conservation of this mountain. In 1932, a project to build up a cableway to the summit, designed by the engineer Antoni Parietti Coll (1899–1979), was proposed. The planned cableway was designed to run a length of 2016 m to the top of Puig Major for 25 passengers per trip as a part of a large project to build an astronomical observatory on the summit, facilities for snow sports, and a restaurant. The project was presented at the Main Theater (Teatre Principal) of Palma in 1934, arousing substantial popular support, after permission granted by the Ministry of Public Works (Rodas, 2009Rodas, G. 2009. El funicular del Puig Major cumple 75 años. Diario de Mallorca, April 19, 2009. Retrieved June 26, 2016, from http://www.diariodemallorca.es/actual/2009/04/19/funicular-puig-major-cumple-75-anos/455703.html). In June 1936, the bottom platform was erected (it is located at km 2.2 of the road MA-2141 to Sa Calobra, 723 m a.s.l.), but the project was interrupted one month later when the Spanish Civil War broke out. At the end of the war (1939), Parietti tried to restart the project with the German firm Bleichord-Zueg. However, this relationship was again interrupted, this time by World War II (Rodas, 2009Rodas, G. 2009. El funicular del Puig Major cumple 75 años. Diario de Mallorca, April 19, 2009. Retrieved June 26, 2016, from http://www.diariodemallorca.es/actual/2009/04/19/funicular-puig-major-cumple-75-anos/455703.html). In the 1950s, when the cableway project was already discarded, Parietti managed to get permission to build a toll road up to the summit, but this new project was shattered as a result of the Spain–USA Defensive Agreement of 26th September 1953 (Delgado, 2003Delgado, L. 2003. ¿El “amigo americano”? España y Estados Unidos durante el franquismo. Studia Historica. Historia Contemporánea 21: 231–276. ), which allowed to set up the “16th USAT Communications Region Tropospheric Station” on the mountain (Moragues et al., 2008Moragues, E., Mayol, J. & Sáez, L. 2008. Flors del Puig Major. Ed. Perifèric, Palma de Mallorca.). An access road was built and the summit was blown up in 1958 losing 9 m of altitude (Moragues et al., 2008Moragues, E., Mayol, J. & Sáez, L. 2008. Flors del Puig Major. Ed. Perifèric, Palma de Mallorca.) to enable the installation of the radar facilities. The embankments of the road and the blowing up of the summit buried large areas, causing impacts on populations of endemic and rare species (Sáez & Rosselló, 2000Sáez, L. & Rosselló, J. A. 2000. A new species of Agrostis (Gramineae) in the A. alpina complex. Botanical Journal of the Linnean Society 133: 359–370. https://doi.org/10.1111/j.1095-8339.2000.tb01551.x, 2001Sáez, L. & Rosselló, J. A. 2001. Llibre vermell de la flora vascular de les Illes Balears. Conselleria de Medi Ambient (Govern de les Illes Balears), Palma de Mallorca., 2003Sáez, L. & Rosselló, J. A. 2003. Agrostis barceloi L. Sáez & Rosselló. In: Bañares, A., Blanca, G., Güemes, J., Moreno, J. C. & Ortiz, S. (Eds.), Atlas y libro rojo de la flora vascular amenazada de España. Táxones prioritarios. Dirección General de Conservación de la Naturaleza, Madrid: 82–83. ; López-Pujol et al., 2013López-Pujol, J., Martinell, M. C., Massó, S., Blanché, C. & Sáez, L. 2013. The ‘paradigm of extremes’: extremely low genetic diversity in anextremely narrow endemic species, Coristospermum huteri (Umbelliferae). Plant Systematics and Evolution 299: 439–446. https://doi.org/10.1007/s00606-012-0732-3; Massó et al., 2016Massó, S., López-Pujol, J., López-Alvarado, J., Blanché, C. & Sáez, L. 2016. One species, one genotype: no genotypic variability in the extremely narrow endemic tetraploid Agrostis barceloi (Gramineae). Plant Systematics and Evolution 302: 609–615. https://doi.org/10.1007/s00606-016-1283-9). This severe impact to the summit of the mountain in 1958 coincided with the birth of the first definite actions of conservation of threatened plants in the Balearic Islands. Shortly before the blown up of the summit, two Majorcan naturalists (Guillem Colom and Jeroni Orell) transplanted specimens of several endemic species to avoid their probable extinction (Moragues et al., 2008Moragues, E., Mayol, J. & Sáez, L. 2008. Flors del Puig Major. Ed. Perifèric, Palma de Mallorca.). Some of these new populations still persist today (e.g. Ranunculus weyleri Marès in Coma de n’Arbona). Fortunately, the situation is now much more favourable from a conservation point of view: the Spanish Ministry of Defence has reduced significantly the size of the installations and it is actively involved in the conservation of the natural heritage of the mountain (Government of Spain, 2010Gobierno de España 2010. Resolución 420/38087/2010, de 29 de abril, de la Secretaría General Técnica, por la que se publica el Convenio de colaboración, entre el Ministerio de Defensa y la Consejería de Medio Ambiente de la Comunidad Autónoma de las Illes Balears, para la conservación de la flora vascular en terrenos de Puig Major (escuadrón de vigilancia aérea nº 7 de Soller-Mallorca). Boletín Oficial del Estado 2010(116): 42081–42086. ).
The case of Puig Major constitutes a vivid example of the effects of military occupation, which may be initially shocking, but at long term resulted positive by avoiding further irreversible alterations (urbanization, tourism facilities, and human frequentation). This situation also occurred in other outstanding areas of the Balearic Islands, such as the archipelago of Cabrera. It is an uninhabited group of islands located off the southern coast of Mallorca, which includes some extremely rare endemic plants [Rubia caespitosa (Font Quer & Marcos) Rosselló—restricted to Cabrera, Beta maritima L. subsp. marcosii (O. Bolòs & Vigo) Juan & M. B. Crespo, Medicago citrina (Font Quer) Greuter, Cymbalaria fragilis (Rodr.) A. Chev., etc.]. The main island was used to house French prisoners during the Napoleonic Wars (1809–1814) becoming the first concentration camp in the history of the world (Pellisier & Phelipeau, 1980Pellisier, P & Phelipeau, J. 1980. Los franceses de Cabrera. Ed. Aucadena, Palma de Mallorca. ). Of 9000 prisoners sent to Cabrera, only 3600 survived. Today this area is a National Park since 1991 and its previous military occupation (1916–1991) prevented urbanization and tourist exploitation in the 1980s, despite the damages caused by military exercises (GOB, 1990GOB [Grup Balear d’Ornitologia i defensa de la Naturalesa] 1990. L’Arxipèlag de Cabrera, un parc nacional en litigi. Ed. Moll, Palma de Mallorca. ).
Sovereign Base Areas of Akrotiri and Dhekelia (Cyprus)
The creation of the British Sovereign Base Areas (SBAs) of Akrotiri and Dhekelia was part of the 1959 agreements for Cypriot independence. In 1960, two separate areas in the southern part of the island were ceded to the UK: Akrotiri (“Western SBA”) and Dhekelia (“Eastern SBA”), which together occupy 254 km2 (i.e. accounting for 2.7% of the total land of Cyprus; Fig. 2). Although being a training area for the British armed forces remains as the primary function of the SBAs, the UK is responsible of protecting their biodiversity (Dodds et al., 2015Dodds, K., Jensen, R. B. & Constantinou, C. M. 2015. Signposts: Cyprus, UK, and the future of the SBAs. The RUSI Journal 160: 36–45. https://doi.org/10.1080/03071847.2015.1102542). The Environmental Department of the SBAs Administration, established in 2002, is in charge of all environmental issues of both SBAs. In addition to their regulatory duties (e.g. enforcing environmental laws, setting up and monitoring PAs), the department is encouraging good ecological and conservation practices among the military (http://www.sbaadministration.org/index.php/environmental).
Despite the small size of these UK enclaves, they harbor rich plant diversity with 328 vascular plant species (Churchyard et al., 2016Churchyard, T., Eaton, M. A., Havery, S. et al. 2016. The biodiversity of the United Kingdom’s Overseas Territories: a stock take of species occurrence and assessment of key knowledge gaps. Biodiversity and Conservation 25: 1677–1694. https://doi.org/10.1007/s10531-016-1149-z); that is, about 16.6% of Cypriot plant species. Although there are no endemic species to the SBAs, several Cypriot endemic species occur, including the second largest population of Ophrys kotchyi, as well as localities of Serapias aphroditae P. Delforge and Taraxacum aphrogenes Meikle (Tsintides et al., 2007Tsintides, T., Christodoulou, C. S., Delipetrou, P. & Georghiou, K. (Eds.) 2007. The red data book of the flora of Cyprus. Cyprus Forestry Association, Lefkosia.). The SBAs also harbor the only Cypriot populations of several species, including Cistanche phelypaea (L.) Cout., Cladium mariscus (L.) Pohl, Convolvulus lineatus L., Coronilla repanda (Poir.) Guss. subsp. repanda, Ipomoea sagittata Poir., Isolepis cernua (Vahl) Roem. & Schult., Linum maritimum L., Lotus cytisoides L., and Serapias parviflora Parl. (Tsintides et al., 2007Tsintides, T., Christodoulou, C. S., Delipetrou, P. & Georghiou, K. (Eds.) 2007. The red data book of the flora of Cyprus. Cyprus Forestry Association, Lefkosia.). Most of these species are located in the Akrotiri Salt Lake (or on its surroundings), which gained international recognition as a Ramsar site in 2003 (http://www.ramsar.org/es/akrotiri). In addition to this, the SBAs Administration has also recently designated up to five Special Areas of Conservation (SACs) to support the existing Natura 2000 network in Cyprus (SBAA, 2015SBAA [Sovereign Base Areas Administration] 2015. Frequently Asked Questions on the designation of Special Areas of Conservation within the Sovereign Base Areas of Akrotiri and Dhekelia. The SBA Administration, Episkopi. Retrieved June 29, 2016, from http://www.sbaadministration.org/images/AEEIC/sac/20151217-ECO_SACs_FAQs_AECO.pdf).
Shoalwater Bay Military Training Area (Queensland, Australia)
Shoalwater Bay Military Training Area (SWBTA) was set up in 1965 with the purchase of over 4500 km2 (of these, about 2900 km2 are terrestrial and 1600 km2 are marine) by the Australian Department of Defence on the Capricorn Coast (central Queensland; Fig. 2). SWBTA is made up of mostly pristine aquatic and marine environments, encompassing areas of the Great Barrier Reef, as well as freshwater and intertidal wetlands (Bowett et al., 2012Bowett, J., Davidson, A. & Danvers, T. 2012. Shoalwater Bay Training Area: capability, conservation and collaboration. In: Figgis, P., Fitzsimons, J. & Irving, J. (Eds.), Innovation for 21st century conservation. Australian Committee for IUCN, Sydney: 142–147.). This makes this area very valuable as a military training area, because realistic maritime and amphibious defense activities can be conducted (Wark & Verrier, 2002). At present, it is considered Australia’s most important area for the conduct of Royal Australian Army, Navy and Air Force combined exercises, and it also serves as a training area for joint exercises with several allies including United States, New Zealand, and Singapore (Bowett et al., 2012Bowett, J., Davidson, A. & Danvers, T. 2012. Shoalwater Bay Training Area: capability, conservation and collaboration. In: Figgis, P., Fitzsimons, J. & Irving, J. (Eds.), Innovation for 21st century conservation. Australian Committee for IUCN, Sydney: 142–147.).
The use of this area as a military training facility has undoubtedly allowed its preservation in an almost pristine state. The areas that were already grazed (about 4%) or selectively logged (22%) prior to acquisition by the government have regenerated well (Department of Defence, 2009Department of Defence 2009. State of the environment report for Shoalwater Bay Training Area 2008. Department of Defence (Australian Government), Canberra.). Gold mining activities, which were common in the area, also stopped when the military took over the place. Commitment of the military with the conservation of the area’s natural heritage dates back to late 1960s—shortly after the training area was established, the first “Ecological Management Plan” was implemented; cooperation with CSIRO, the federal government agency for scientific research in Australia, also began at that time (Bowett et al., 2012Bowett, J., Davidson, A. & Danvers, T. 2012. Shoalwater Bay Training Area: capability, conservation and collaboration. In: Figgis, P., Fitzsimons, J. & Irving, J. (Eds.), Innovation for 21st century conservation. Australian Committee for IUCN, Sydney: 142–147.). Since 1994, conservation in SWBTA is regarded as having equal significance than military use, following the recommendation of the Commonwealth Commission of Inquiry into Shoalwater Bay (Department of Defence, 2009Department of Defence 2009. State of the environment report for Shoalwater Bay Training Area 2008. Department of Defence (Australian Government), Canberra.). SWBTA is, thus, regarded as one of the best conserved areas of Australia (included in the Commonwealth Heritage List since 2004), having a high diversity of species but also well-preserved ecosystems. For example, SWBTA constitutes the largest remaining area of sub-tropical coastal heathland on the Australian east coast—an ecosystem poorly protected in Australian PAs and subject to major human modification outside them (Zentelis & Lindenmayer, 2015Zentelis, R. & Lindenmayer, D. 2015. Bombing for biodiversity—enhancing conservation values of military training areas. Conservation Letters 8: 299–305. https://doi.org/10.1111/conl.12155). Large sections of the military training area are part of internationally recognized PAs; the marine areas are part of the Great Barrier Reef World Heritage Area (which is the third largest World Heritage Area in the world), whereas most of the Ramsar site “Shoalwater and Corio Bays” falls within SWBTA (Department of Defence, 2009Department of Defence 2009. State of the environment report for Shoalwater Bay Training Area 2008. Department of Defence (Australian Government), Canberra.). In addition to natural heritage, SWBTA is also preserving several assets of historic and cultural heritage. The recommendations of the Commonwealth Commission of Inquiry into Shoalwater Bay of 1994 also pointed out the richness in archaeological, cultural, and spiritual sites and values of the area, including Aboriginal settlements, places associated with explorers James Cook and Matthew Flinders, and subsequent European settlements; all of them are effectively protected by the Australian Environment Protection and Biodiversity Conservation Act of 1999 and their subsequent amendments (Department of Defence, 2009Department of Defence 2009. State of the environment report for Shoalwater Bay Training Area 2008. Department of Defence (Australian Government), Canberra.).
MILITARY RELICSTop
Spanish castles and fortifications
The territory of the present Spain has a long history of wars, with construction of castles having mirrored this. Among the over 2500 castles and fortifications spread along the country, we are aware of at least three of them—cited as examples—having contributed to the preservation of plant species, mostly in a way of “concerted” conservation of natural heritage and historical relics.
Cornatel Castle (Priaranza del Bierzo, Castile and Leon, Spain)
This castle (Fig. 2), which dates of 9th century AD (and which on 13th century was transferred to the Templar Knights) harbors a small population of the threatened and narrow endemic Petrocoptis viscosa Rothm. [≡ P. pyrenaica (Bergeret) A. Braun ex Walp. subsp. viscosa (Rothm.) P. Monts. & Fern. Casas] in its walls and on nearby outcrops. Petrocoptis is a flagship genus in Spain because it is one of the ca. 20 endemic genera of the Iberian Peninsula (Moreno, 2011Moreno, J. C. 2011. La diversidad florística vascular española. Memorias de la Real Sociedad Española de Historia Natural 9: 75–107. Mortimer, 2009Mortimer, I. 2009. 1415: Henry V’s year of glory. Random House, London.). Petrocoptis viscosa is only known from three populations with an area of occupancy of just 0.10 km2; the Cornatel Castle population has about 370 individuals (about 10% of the total census size; Miranda et al., 2014Miranda, B., Acedo, C. & Llamas, F. 2014. Petrocoptis pyrenaica subsp. viscosa. Fichas con recopilación de información sobre las especies incluidas en el Decreto 63/2007. Consejería de Medio Ambiente (Junta de Castilla y León), Valladolid.). Although the species is included in the protection list of Castile and Leon region (Junta de Castilla y León, 2007Junta de Castilla y León 2007. Decreto 63/2007, de 14 de junio, por el que se crean el Catálogo de Flora Protegida de Castilla y León y la figura de protección denominada Microrreserva de Flora. Boletín Oficial de Castilla y León 2007(119): 13197–13204. ), no specific measures to preserve this population have been implemented yet (C. Acedo & F. Llamas, pers. comm.). Therefore, the preservation of the castle ruins can be regarded as the only in situ conservation measure; fortunately, the castle was declared in 1949 as “Heritage of Cultural Interest”, and in 2005–2006 it was completely restored (with special care to avoid any damage to the plant population; Fidalgo, 2006Fidalgo, C. 2006. Sobrevivir a la leyenda. La Torre del Homenaje, sin socavón. Diario de León, April 16, 2006. Retrieved July 4, 2016, from http://www.diariodeleon.es/noticias/bierzo/sobrevivir-leyenda-torre-homenaje-sin-socavon_254652.html). The legal preservation of the castle since middle 20th century, unfortunately, was not enough to prevent the loss of another Petrocoptis species (P. grandiflora Rothm.) that also lived on its walls. The small population that was still present at the beginning of 1990s (Guitián et al., 1993Guitián, J., Sánchez, J. M. & Guitián, P. 1993. Biología y conservación de Petrocoptis grandiflora en el Noroeste Ibérico. Botanica Complutensis 18: 123–128.) is now extinct, probably due to over-collection (Carbajal et al., 2010Carbajal, R., Gómez, M. A., Navarro, L., Rodríguez, J. & Serrano, M. 2010. Petrocoptis grandiflora Rothm. In: Bañares, A., Blanca, G., Güemes, J., Moreno, J. C. & Ortiz, S. (Eds.), Atlas y libro rojo de la flora vascular amenazada de España. Addenda 2010. Dirección General para la Biodiversidad – Sociedad Española de Biología de la Conservación de Plantas, Madrid: 122–123.).
Xàtiva Castle (Xàtiva, Valencian Community, Spain)
Located in La Costera county (Alacant Province; Figs. 2 and 3), the Xàtiva Castle consists actually in a 2-unit structure (called Castell Menor and Castell Major) of very old origins (including Iberian Pre-Roman, Roman, Muslim, Medieval and Baroque structures), with a very relevant role as strategic point in Hannibal campaigns (2nd century BC), Al-Andalus period (9–13th centuries AD) or Succession War (18th century AD, the city destroyed by order of the Spanish king Felipe V) as well as Aragon Crown State Prison for notorious prisoners (Hernàndez, 2003Hernàndez, F. X. 2003. Història militar de Catalunya 3. Rafael Dalmau, Barcelona.; Alcoberro, 2006Alcoberro, A. (Coord.) 2006. Catalunya durant la Guerra de Successió (1–3). Ara Llibres, Badalona. ). The inexpugnability of that fortress is mainly due to its position at the top of a mountain crest, surrounded by antique long walls (some parts more than 1000 years old are still remaining) built from the same calcareous materials than the surrounding cliffs, thus sharing the same rupicolous vegetation, including some rocky specialist endemic and threatened species (Fig. 3).
The Valencian Microreserves Network consists of a number of protected small spaces created in 1998 with the support of the LIFE European Union programme (see Laguna et al., 2004Laguna, E., Deltoro, V. I., Pérez-Botella, J., Pérez-Rovira, P., Serra, L., Olivares, A. & Fabregat, C. 2004. The role of small reserves in plant conservation in a region of high diversity in eastern Spain. Biological Conservation 119: 421–426. https://doi.org/10.1016/j.biocon.2004.01.001). The good conservation status of the chasmophytic vegetation harbored by both natural vertical and castle walls (ensured by its declaration as “Heritage of Cultural Interest” in 1931) allowed the creation, in 1999, of a microreserve (“Microreserva de Flora”) by the Valencian Government, also including historic architectural elements with a total surface of only 3.37 ha (enough however to include a substantial part of the needed habitat for rocky specialist endemics). The good state of such isolated calcareous outcrops and cliffs allowed further recognition of this space as SCI (Site of Community Importance) to include well preserved dry Mediterranean calcareous pastures (EU Habitat *6210) with the endemic and protected campion Silene diclinis (Lag.) M. Laínz [EN B1ab(iii,v)+2ab(iii,v); C1; Montesinos & Güemes, 2006Montesinos, D. & Güemes, J. 2006. Silene diclinis. The IUCN Red List of Threatened Species 2006: e.T61640A12531397. IUCN, Gland. Retrieved June 30, 2016, from http://dx.doi.org/10.2305/IUCN.UK.2006.RLTS.T61640A12531397.en], as well as a good representation of chasmophytic vegetation in walls (EU Habitat 8210) with other endemic taxa as Sarcocapnos saetabensis Mateo & Figuerola (Fig. 3, epithet from Saiti, the Iberian and Saetabis, the Latin Roman, both former names of Xàtiva), Saxifraga corsica (Ser.) Gren. & Godr. subsp. cossoniana (Boiss. & Reut.) D. A. Webb, or Chaenorhinum origanifolium (L.) Kostel. (Laguna, 1998Laguna, E. (Coord.) 1998. Flora endémica, rara o amenazada de la Comunidad Valenciana. Conselleria de Medi Ambient (Generalitat Valenciana), Valencia.). These species are today well preserved under the European Union legal figure of SAC (Special Area of Conservation) (Generalitat Valenciana, 2016Generalitat Valenciana 2016. Espais naturals protegits – Xàtiva. Generalitat Valenciana, València. Retrieved June 12, 2016, from http://www.argos.gva.es/bdmun/pls/argos_mun/DMEDB_MUNDATOSESPNATURALES.DibujaPagina?aNMunId=46145&aVLengua=V) and an extended (up to 4.89 ha) new delimitation of the microrserve Serra del Castell de Xàtiva (Generalitat Valenciana, 2011Generalitat Valenciana 2011. Ordre 2/2011, de 24 de gener, de la Conselleria de Medi Ambient, Aigua, Urbanisme i Habitatge, per la qual es declaren sis noves microreserves de flora a la província de València i es modifiquen les ordres de declaració de microreserves de flora de 4 de maig de 1999, 6 de novembre de 2000, 22 d’octubre de 2002 i 24 d’octubre de 2003. Diari Oficial de la Comunitat Valenciana 6450: 4794–4811. ). The main targeted species (Silene diclinis) has been object of multiple monitoring, conservation and management efforts, many of them in the Xàtiva castle area (Aguilella et al., 2009Aguilella, A., Fos, S. & Laguna, E. (Eds.) 2009. Catálogo valenciano de especies de flora amenazadas. Conselleria de Medi Ambient, Aigua, Urbanisme i Habitatge (Generalitat Valenciana), Valencia.).
Sant Ferran Fortress (Figueres, Catalonia, Spain)
One of the most endangered species of NE Spain is a small campion, Silene sennenii Pau (Fig. 4), which has the particularity that shows nocturnal pollination (Martinell et al., 2010Martinell, M. C., Dötterl, S., Blanché, C., Rovira, A., Massó, S. & Bosch, M. 2010. Nocturnal pollination of the endemic Silene sennenii (Caryophyllaceae): an endangered mutualism? Plant Ecology 211: 203–218. https://doi.org/10.1007/s11258-010-9785-y). The species has declined due to the loss and fragmentation of its natural habitat (dry grasslands) as consequence of its conversion into irrigated croplands and the expansion of urban and industrial areas. At present, only five populations of the species remain, with fewer than 5000 individuals in total and a mere 0.5 km2 of area of occupancy (Martinell et al., 2010Martinell, M. C., Dötterl, S., Blanché, C., Rovira, A., Massó, S. & Bosch, M. 2010. Nocturnal pollination of the endemic Silene sennenii (Caryophyllaceae): an endangered mutualism? Plant Ecology 211: 203–218. https://doi.org/10.1007/s11258-010-9785-y). The low levels of genetic diversity and the observed disruption of the plant-pollinator mutualisms in some populations can be interpreted as the effects of such habitat changes (López-Pujol et al., 2007López-Pujol, J., Font, J., Simon, J. & Blanché, C. 2007. Can the preservation of historical relicts permit the conservation of endangered plant species? The case of Silene sennenii (Caryophyllaceae). Conservation Genetics 8: 903–912. https://doi.org/10.1007/s10592-006-9245-3; Martinell et al., 2010Martinell, M. C., Dötterl, S., Blanché, C., Rovira, A., Massó, S. & Bosch, M. 2010. Nocturnal pollination of the endemic Silene sennenii (Caryophyllaceae): an endangered mutualism? Plant Ecology 211: 203–218. https://doi.org/10.1007/s11258-010-9785-y). However, there is a hope for this threatened species: ca. 70% of the total population (and, very importantly, all the alleles detected for the species) is dwelling in and around a fortress built in the 18th century, a circumstance which might have facilitated the preservation of a relatively large population (of over 3000 plants; Martinell, 2010Martinell, M. C. 2010. Biología de la conservación de especies amenazadas de área de distribución restringida en Cataluña. PhD Thesis, Universitat de Barcelona, Barcelona. ) until now. The Sant Ferran Fortress is considered the largest 18th century fortress in Europe, encompassing over 50 ha (Figs. 2 and 4). Built to prevent future French invasions, the fortification became a military prison during the 20th century; it was not until recent times (1997) that the castle became open to public. At present, even though the fortress has no military use, the Spanish Army maintains ownership, and a public consortium formed by the Ministry of Defence, the regional government, and the local authorities is now in charge of the management of the building and the surrounding lands. Despite Silene sennenii is a species included both in the regional (Generalitat de Catalunya, 2008Generalitat de Catalunya 2008. Decret 172/2008, de 26 d’agost, de creació del Catàleg de flora amenaçada de Catalunya. Diari Oficial de la Generalitat de Catalunya 5204: 65881–65895. ) and state (Gobierno de España, 2015Gobierno de España 2015. Orden AAA/1771/2015, de 31 de agosto, por la que se modifica el anexo del Real Decreto 139/2011, de 4 de febrero, para el desarrollo del Listado de Especies Silvestres en Régimen de Protección Especial y del Catálogo Español de Especies Amenazadas. Boletín Oficial del Estado 2015(211): 77925–77929. ) lists of protected plants, neither any of the extant populations are included within PAs nor other in situ conservation measures are in place (although occasional actions—including controlled grazing by sheeps—as well as ex situ conservation measures have been undertaken). Thus, the preservation of the castle (it was declared in 1949 as “Heritage of Cultural Interest”) and its surrounding lands (individuals of Silene sennenii occur both in the fortress glacis and covered way; Fig. 4) in a more or less intact state seems to be the only effective way to ensure the long-term survival of the species.
|
Figure 4. (A), aerial image of Sant Ferran Fortress; (B), Silene sennenii; (C), Sant Ferran Fortress glacis, where Silene sennenii grows. All the pictures were taken by S. Massó except A (photograph: ©Institut Cartogràfic de Catalunya).

[View full size] [Descargar tamaño completo] |
|
Longues-sur-Mer Battery (and other sites related to 1944 Normandy landings) (Normandy, France)
This World War II artillery battery formed part of Nazi Germany “Atlantic Wall”, a massive coastal defense system that spanned from the North Cape in Norway to the French/Spanish border. The Longues-sur-Mer Battery, situated between Omaha and Gold beaches (two of the landing sites of the Day-D) in Normandy (Fig. 2) and completed in April of 1944, mainly consisted of four heavy guns protected by concrete casemates and a command post (Fig. 5). This is an excellent example of concerted conservation of natural and historic heritage in which both items are enjoying legal protection (and thus, the site can be regarded as a de iure nature reserve). Whereas the site (of 60 ha in total) is protected since 1967 (in fact, by an old but pioneering law of 1930—now repealed—whose purpose was the organization of natural sites with artistic, historical, legendary or picturesque character), in 2001 it was upgraded to be a Monument Historique, thus enjoying the highest degree of protection in France. Regarding its ecological value, a large part of the site is protected since 1984, when ca. 25 ha were acquired by the Conservatoire du littoral (Conservatoire du littoral, 2016aConservatoire du littoral 2016a. Batterie de Longues. Conservatoire du littoral, Rochefort. Retrieved March 12, 2016, from http://www.conservatoire-du-littoral.fr/siteLittoral/126/28-batterie-de-longues-14_calvados.htm), a French public organization and an IUCN member that is mainly devoted to ensure the preservation of coastal and lakeshore areas. The site is regarded as an IUCN category IV protected area (MNHN, 2016MNHN [Muséum National d’Histoire Naturelle] 2016. FR1100039 – Batterie de Longues. MNHN, Paris. Retrieved March 12, 2016, from https://inpn.mnhn.fr/espace/protege/FR1100039), with the spectacular cliffs of the area being home of the regionally-protected, narrow endemic Tephroseris helenitis (L.) B. Nord. subsp. candida (Corb.) B. Nord. (with an estimated total population of less than 5000 individuals; Housset & Lemire, 2009Housset, P. & Lemire, J. 2009. Bilan des stratégies minimales régionales de conservation – Région Haute-Normandie. Le Jouet du Vent 21: 6.).
|
Figure 5. Location and images of the seven sites related to the Normandy Landings (1944, World War II) mentioned in the text. (1), Utah Beach; (2), Pointe du Hoc; (3), Omaha Beach; (4), Mont-Castel; (5), Les Fonderies; (6), Longues-sur-Mer Battery (above: panel of the Conservatoire du littoral; below: one of the four heavy guns in its casemate); (7), Merville Battery. All the pictures were taken by J. López-Pujol except 4 (photograph: © François Levalet).
[View full size] [Descargar tamaño completo] |
|
Notably, there are several sites in addition to the Longues-sur-Mer Battery that enjoy a similar typology of concerted conservation in the same area, mostly related to the Nazi fortifications and the Day-D landings (Fig. 5). Conservatoire du littoral is protecting other World War II historical sites such as Omaha and Utah beaches (the two most famous landing beaches of D-Day, both protected as historical sites in recent years), Merville Battery (another major battery of the Atlantic Wall, and which, as for the case of Longues-sur-Mer Battery, is listed as Monument Historique), Pointe du Hoc (a promontory that was fortified by the German army with concrete casemates and gun pits; protected since the 1950s), or Les Fonderies (where the famous Winston Churchill’s artificial “Mulberry” harbor was built; protected since early 2000s). Two of these sites (Pointe du Hoc and Omaha Beach) also enjoy protection as historical sites from the American Government (the first site and part of the second are managed by the American Battle Monuments Commission; Conservatoire du littoral, 2015Conservatoire du littoral 2015. Plans de gestion du Conservatoire du littoral. Sites des dunes et falaises du Bessin – Calvados. Conservatoire du littoral Délégation Normandie, Caen. Retrieved March 12, 2016, from http://www.conservatoire-du-littoral.fr/include/viewFile.php?idtf=6510&path=78%2F6510_421_Dunes_et_falaises_du_Bessin_opti.pdf). Recently, several strategies aimed to implement a common management plan are already in place or under preparation, including the plan for the preservation of the whole Bessin coastal area by Conservatoire du littoral (that will include most of the above mentioned sites; Conservatoire du littoral, 2015Conservatoire du littoral 2015. Plans de gestion du Conservatoire du littoral. Sites des dunes et falaises du Bessin – Calvados. Conservatoire du littoral Délégation Normandie, Caen. Retrieved March 12, 2016, from http://www.conservatoire-du-littoral.fr/include/viewFile.php?idtf=6510&path=78%2F6510_421_Dunes_et_falaises_du_Bessin_opti.pdf) and the declaration of up to 11 Normandy-landings historical places as forming the “Normandy 1944” Great Sight-Seeing of France (Grands Sites de France); this latter plan is mainly aimed to ensure that tourism development is compatible with the preservation of natural areas (Duval & Gauchon, 2007Duval, M. & Gauchon, C. 2007. Analyse critique d’une politique d’aménagement du territoire, les Opérations Grands Sites. Annales de Géographie 654: 147–168. https://doi.org/10.3917/ag.654.0147). Remarkably, Mont-Castel, a small hill which is one of the sites to be protected by the common management plan of Conservatoire du littoral, contains vestiges of military facilities from at least four historical periods (Lefort et al., 2012Lefort, A., Marcigny, C., Giraud, P. & Guihard, P.-M. 2012. L’oppidum du Mont-Castel (Port-en-Bessin-Huppain, Calvados). Premiers résultats. Revue Archéologique de l’Ouest 29: 107–132. https://doi.org/10.4000/rao.1801; Conservatoire du littoral, 2016bConservatoire du littoral 2016b. Mont Castel. Conservatoire du littoral, Rochefort. Retrieved March 14, 2016, from http://www.conservatoire-du-littoral.fr/siteLittoral/124/28-mont-castel-14_calvados.htm): Late Bronze Age (a rampart), Ancient Rome (militaria), late 17th century (an artillery tower, listed as Monument Historique), and World War II (casemates).
CONCLUSIONS AND RECOMMENDATIONSTop
War is not the answer. But in a changing world, with conservation biologists committed with biodiversity conservation as a crisis-oriented discipline (Soulé, 1985Soulé, M. I. 1985. What is conservation biology? BioScience 35: 727–734. https://doi.org/10.2307/1310054), an accurate examination of all the available resources allowing the long-term viability of species and ecosystems is a challenge and a major concern. War (and related activities and resources) must also be considered if any of the derived consequences can be employed in plant conservation to, at least, partially compensate its destructive effects on man and biosphere. Other groups of organisms have obtained unexpected insights for conservation purposes from military facts; for instance, a 6-year pause in commercial fishing caused by World War II helped cod, haddock and whiting populations in Europe’s North Sea recover from years of pre-war exploitation, according to a new analysis. The “accidental” reserve suggests that cold-water fish stocks could benefit from modern marine protected areas (Beare et al., 2010Beare, D., Hölker, F., Engelhard, G. H., McKenzie, E. & Reid, D. G. 2010. An unintended experiment in fisheries science: a marine area protected by war results in Mexican waves in fish numbers-at-age. Naturwissenschaften 97: 797–808. https://doi.org/10.1007/s00114-010-0696-5). Based on the case-studies examined and on other information gathered by us, the following conclusions and recommendations can be made:
- Although difficult to accept in terms of our current life believes, while wars (declared or not) exist, their biodiversity impacts can (and have to) be minimized and prevented. The application of weapons, the destruction of structures and oil fields, fires, military transport movements, and chemical spraying are all examples of the destroying impact that war may have on the environment. Air, water and soil are polluted, men and animals are killed, and numerous health affects occur among those still living (Enzler, 2006Enzler, S. M. 2006. Environmental effects of warfare. Lenntech BV Delft. Retrieved July 3, 2016, from http://www.lenntech.com/environmental-effects-war.htm). In consequence, international provisions have to be taken into account, particularly that included in the 1992 Rio Declaration: “Warfare is inherently destructive of sustainable development. States shall therefore respect international law providing protection for the environment in times of armed conflict and cooperate in its further development, as necessary”.
- To reinforce this principle, complementary tools have been developed by international bodies. UN promotional “days” and public education initiatives can be taken as examples: on 5 November 2001, the UN General Assembly declared 6 November of each year as the International Day for Preventing the Exploitation of the Environment in War and Armed Conflict (Resolution 56/4; http://www.un.org/en/ga/search/view_doc.asp?symbol=A/RES/56/4). The United Nations attaches great importance to ensuring that action on the environment is part of conflict prevention, peacekeeping and peacebuilding strategies—because there can be no durable peace if the natural resources that sustain livelihoods and ecosystems are destroyed (http://www.un.org/en/events/environmentconflictday/).
- Once a conflict has finished, accurate assessments of short-, mid- or long-term consequences on plant diversity of warfare are mandatory. Complexity of interactions and side effects of distinct alterations to different biodiversity compartments have to be carefully checked (e.g. a decline of large mammals due to Mozambican Civil War has led to an increase in tree cover due to a decrease in grazing; cf. Sugden, 2016Sugden, A. M. 2016. Ecological legacy of a civil war. Science 351: 572–573. https://doi.org/10.1126/science.351.6273.572-a), as actually needed in any management or conservation standard plan.
- Restoration of destroyed habitats, ecosystems and species by means of ad-hoc recovery plans (design, implementation and long-term monitoring appropriately subsidized) should be an essential part of the post-war reconstruction projects.
- Abandoned battlefields, military areas, airfields, communications complexes, isolated castles, fortresses, forts, island bases, etc. are lands, sometimes of very important extension, commonly of restricted access. They have preserved natural habitats (sometimes during long time) resulting in de facto nature reserves, by simply maintaining wide and remote lands out from development pressures (one of the main threats to plant diversity conservation; Salafsky et al., 2008Salafsky, N., Salzer, D., Stattersfield, A. J. et al. 2008. A standard lexicon for biodiversity conservation: unified classifications of threats and actions. Conservation Biology 22: 897–911. https://doi.org/10.1111/j.1523-1739.2008.00937.x; Sáez et al., 2010Sáez, L., Aymerich, P. & Blanché, C. 2010. Llibre vermell de plantes vasculars endèmiques i amenaçades de Catalunya. Argania Editio, Barcelona. ). Hundreds of examples could be documented. As many of these zones are public domains, states and other governmental entities should have administrative rights to easily convert (totally or partially) those areas in de iure nature reserves according to the appropriate prioritization processes, in relatively short time periods. The cases of Xàtiva Castle and Cabrera Archipelago stated above are good examples of this procedure, but thousands of additional initiatives could be found.
- Specific programs to promote the transformation of military zones into conservation areas could be launched, in some parallel ways to other sectorial programs exploiting confluence of biodiversity conservation interests with complementary areas (as the Delos Initiative, promoted by the IUCN Specialist Group on the Cultural and Spiritual Values of Protected Areas to promote the integrated management of the natural and cultural heritage on the sacred natural sites in developed countries; MEDINA, 2016MEDINA [Mediterranean Institute for Nature and Anthropos] 2016. The Delos Initiative. MEDINA, Athens. Retrieved July 5, 2016, from http://www.med-ina.org/delos/index.htm).
- If de iure conversion of military areas and equipment are not possible at present, specific agreements with public or private organizations to implement conservation projects can be signed. The case of Fort Bragg conservation initiatives, agreed more than 20 years ago and still active, are illustrative of such temporal procedure (although a more stable arrangement in the future could be expected). On the contrary, persistence of practices implying habitat degradation in adverted army installations containing endangered plant populations should be immediately stopped. For instance, modification or translocation of guns and tanks and shooting practices in training military camps containing threatened species should be carefully monitored or even prescribed by nature conservation authorities [there are examples in Spain: the Columbrets Islands, an airforce bombing training area in 1978, is nowadays a nature reserve; or the shooting training in the military zones of Sant Climent Sescebes, which is currently contested by the local residents (Dossiers Crítics, 2004Dossiers Crítics 2004. Les Columbretes: present i passat d’una lluita ecologista. Dossiers Crítics 8. Retrieved July 1, 2016, from http://altrecastello.probeta.net/dossiers/columbre.htm; Sáez et al., 2010Sáez, L., Aymerich, P. & Blanché, C. 2010. Llibre vermell de plantes vasculars endèmiques i amenaçades de Catalunya. Argania Editio, Barcelona. )].
- Historical monuments (architectural heritage) protection can be also coupled with biodiversity conservation if coordinate actions are taken. This strategy, sometimes called “concerted conservation” has proven effective (to both components: monuments and plants) and efficient (economically and biologically) (Liu et al., 2002Liu, H., Xu, Z., Xu, Y. & Wang, J. 2002. Practice of conserving plant diversity through traditional beliefs: a case study in Xishuangbanna, southwest China. Biodiversity and Conservation 11: 705–713. https://doi.org/10.1023/A:1015532230442; López-Pujol et al., 2006López-Pujol, J., Zhang, F.-M. & Ge, S. 2006. Plant biodiversity in China: richly varied, endangered and in need of conservation. Biodiversity and Conservation 15: 3983–4026. https://doi.org/10.1007/s10531-005-3015-2, 2007López-Pujol, J., Font, J., Simon, J. & Blanché, C. 2007. Can the preservation of historical relicts permit the conservation of endangered plant species? The case of Silene sennenii (Caryophyllaceae). Conservation Genetics 8: 903–912. https://doi.org/10.1007/s10592-006-9245-3). On the contrary, uncoupling has demonstrated that historical building conservation actions can also be the origin of plant populations threat or even extinction (see Benito, 2008Benito, J. L. 2008. Conservació concertada de flora i de monuments històrics – Comentari. E-Opinió núm. 37. Portal de Biologia de la Conservació de plantes (Universitat de Barcelona), Barcelona. Retrieved July 3, 2016, from http://hdl.handle.net/2445/27722).
- The traditional reluctance of military personnel to collaborate with conservation organizations or the academic world—as well as in the opposite direction—should be overcome. Recent experiences in several continents such as the participation of the Spanish Army in the recovery plan of the Puig Major Mountain flora, the cooperation of the Australian Army with CSIRO for the conservation of the Shoalwater Bay Military Training Area, or the involvement of the US Army in the management plan for cultural and natural resources for Fort Bragg (there is even a branch of the military base devoted to endangered species; see above) are encouraging practices and show that the world armies may play a role in nature conservation.
Finally, warfare is not the only threat which biodiversity conservation has to fight against. The “sacred” Delphi-Itea olive tree fields escaped from ancient military devastation practices but they entered again however at risk, 26 centuries later, by a contemporary touristic olive-packing unit installation project. Unfortunately, its declaration as protected zone did not avert the destruction of a large part of it by a fire in August 2013 (UNESCO, 1992–2016UNESCO 1992–2016. World Heritage List. Archeological site of Delphi. UNESCO World Heritage Centre, Paris. Retrieved July 2, 2016, from http://whc.unesco.org/en/list/393). Habitat destruction under global change pressures is also an open war to win.
ACKNOWLEDGEMENTSTop
We are indebted to C. Acedo and F. Llamas for providing information on Petrocoptis species from Cornatel Castle (León, Spain), and to F. Levalet for kindly providing a picture of Mont-Castel (Normandy, France).
REFERENCESTop
|
| 01. |
Aguilella, A., Fos, S. & Laguna, E. (Eds.) 2009. Catálogo valenciano de especies de flora amenazadas. Conselleria de Medi Ambient, Aigua, Urbanisme i Habitatge (Generalitat Valenciana), Valencia. |
| 02. |
Alcoberro, A. (Coord.) 2006. Catalunya durant la Guerra de Successió (1–3). Ara Llibres, Badalona. |
| 03. |
Ali, S. H. (Ed.) 2007. Peace Parks: Conservation and conflict resolution. MIT Press, Cambridge. |
| 04. |
Álvarez, M. D. 2003. Forests in the time of violence: conservation implications of the Colombian War. Journal of Sustainable Forestry 16: 49–70. https://doi.org/10.1300/J091v16n03_03 |
| 05. |
Ames, G. J. 2008. The globe encompassed: the age of European discovery, 1500–1700. Prentice Hall, London. |
| 06. |
Amigues, S. 2010. Théophraste. Recherches sur les plantes. À l’origine de la botanique. Éditions Belin, Paris. |
| 07. |
Beare, D., Hölker, F., Engelhard, G. H., McKenzie, E. & Reid, D. G. 2010. An unintended experiment in fisheries science: a marine area protected by war results in Mexican waves in fish numbers-at-age. Naturwissenschaften 97: 797–808. https://doi.org/10.1007/s00114-010-0696-5 |
| 08. |
Benito, J. L. 2008. Conservació concertada de flora i de monuments històrics – Comentari. E-Opinió núm. 37. Portal de Biologia de la Conservació de plantes (Universitat de Barcelona), Barcelona. Retrieved July 3, 2016, from http://hdl.handle.net/2445/27722 |
| 09. |
BGCI [Botanic Gardens Conservation International] 1996. Proposals for the Reconstruction of the Zemaljski Museum and the Botanic Garden, Sarajevo. BGCI News 2(6). Retrieved July 3, 2016, from http://www.bgci.org/worldwide/article/0166/ |
| 10. |
Böer, B. 1993. Anomalous pneumatophores and adventitious roots of Avicennia marina (Forssk.) Vierh. mangroves two years after the 1991 Gulf War oil spill in Saudi Arabia. Marine Pollution Bulletin 27: 207–211. https://doi.org/10.1016/0025-326X(93)90026-G |
| 11. |
Bowett, J., Davidson, A. & Danvers, T. 2012. Shoalwater Bay Training Area: capability, conservation and collaboration. In: Figgis, P., Fitzsimons, J. & Irving, J. (Eds.), Innovation for 21st century conservation. Australian Committee for IUCN, Sydney: 142–147. |
| 12. |
Carbajal, R., Gómez, M. A., Navarro, L., Rodríguez, J. & Serrano, M. 2010. Petrocoptis grandiflora Rothm. In: Bañares, A., Blanca, G., Güemes, J., Moreno, J. C. & Ortiz, S. (Eds.), Atlas y libro rojo de la flora vascular amenazada de España. Addenda 2010. Dirección General para la Biodiversidad – Sociedad Española de Biología de la Conservación de Plantas, Madrid: 122–123. |
| 13. |
Charles, D. 2006. A ‘Forever’ seed bank takes root in the Arctic. Science 312: 1730–1731. https://doi.org/10.1126/science.312.5781.1730b |
| 14. |
Cheng, S. & McBride, J. R. 2006. Restoration of the urban forests of Tokyo and Hiroshima following World War II. Urban Forestry & Urban Greening 5: 155–168. https://doi.org/10.1016/j.ufug.2006.07.003 |
| 15. |
Chung, M. Y., López-Pujol, J. &, Chung, M. G. 2017. The role of the Baekdudaegan (Korean Peninsula) as a major glacial refugium for plant species: A priority for conservation. Biological Conservation 206: 236–248. https://doi.org/10.1016/j.biocon.2016.11.040 |
| 16. |
Churchyard, T., Eaton, M. A., Havery, S. et al. 2016. The biodiversity of the United Kingdom’s Overseas Territories: a stock take of species occurrence and assessment of key knowledge gaps. Biodiversity and Conservation 25: 1677–1694. https://doi.org/10.1007/s10531-016-1149-z |
| 17. |
Conservatoire du littoral 2015. Plans de gestion du Conservatoire du littoral. Sites des dunes et falaises du Bessin – Calvados. Conservatoire du littoral Délégation Normandie, Caen. Retrieved March 12, 2016, from http://www.conservatoire-du-littoral.fr/include/viewFile.php?idtf=6510&path=78%2F6510_421_Dunes_et_falaises_du_Bessin_opti.pdf |
| 18. |
Conservatoire du littoral 2016a. Batterie de Longues. Conservatoire du littoral, Rochefort. Retrieved March 12, 2016, from http://www.conservatoire-du-littoral.fr/siteLittoral/126/28-batterie-de-longues-14_calvados.htm |
| 19. |
Conservatoire du littoral 2016b. Mont Castel. Conservatoire du littoral, Rochefort. Retrieved March 14, 2016, from http://www.conservatoire-du-littoral.fr/siteLittoral/124/28-mont-castel-14_calvados.htm |
| 20. |
Crosby, A. W. 1988. Imperialismo ecológico: la expansión biológica de Europa, 900-1900. Editorial Crítica, Barcelona. |
| 21. |
Davis, V. H. 1998. Warfare and agriculture in Classical Greece (revised ed.). University of California Press, Berkeley & Los Angeles. |
| 22. |
Delgado, L. 2003. ¿El “amigo americano”? España y Estados Unidos durante el franquismo. Studia Historica. Historia Contemporánea 21: 231–276. |
| 23. |
Del Tredici, P., Ling, H. & Yang, G. 1992. The Ginkgos of Tian Mu Shan. Conservation Biology 6: 202–209. https://doi.org/10.1046/j.1523-1739.1992.620202.x |
| 24. |
Department of Defence 2009. State of the environment report for Shoalwater Bay Training Area 2008. Department of Defence (Australian Government), Canberra. |
| 25. |
Determann, J. M. 2015. Researching biology and evolution in the Gulf states – Networks of science in the Middle East. I. B. Tauris, London & New York. |
| 26. |
DeWeerdt, S. 2008. War and the environment. World Watch Magazine 21(1): 14–21. |
| 27. |
Dodds, K., Jensen, R. B. & Constantinou, C. M. 2015. Signposts: Cyprus, UK, and the future of the SBAs. The RUSI Journal 160: 36–45. https://doi.org/10.1080/03071847.2015.1102542 |
| 28. |
Dossiers Crítics 2004. Les Columbretes: present i passat d’una lluita ecologista. Dossiers Crítics 8. Retrieved July 1, 2016, from http://altrecastello.probeta.net/dossiers/columbre.htm |
| 29. |
Draulans, D. & Van Krunkelsven, E. 2002. The impact of war on forest areas in the Democratic Republic of Congo. Oryx 36: 35–40. https://doi.org/10.1017/S0030605302000066 |
| 30. |
Duval, M. & Gauchon, C. 2007. Analyse critique d’une politique d’aménagement du territoire, les Opérations Grands Sites. Annales de Géographie 654: 147–168. https://doi.org/10.3917/ag.654.0147 |
| 31. |
Enzler, S. M. 2006. Environmental effects of warfare. Lenntech BV Delft. Retrieved July 3, 2016, from http://www.lenntech.com/environmental-effects-war.htm |
| 32. |
Fidalgo, C. 2006. Sobrevivir a la leyenda. La Torre del Homenaje, sin socavón. Diario de León, April 16, 2006. Retrieved July 4, 2016, from http://www.diariodeleon.es/noticias/bierzo/sobrevivir-leyenda-torre-homenaje-sin-socavon_254652.html |
| 33. |
Folch, R. 2011. La quimera de créixer. La sostenibilitat en l’era postindustrial. La Magrana, Barcelona. |
| 34. |
Galán de Mera, A. & Sáez, L. 2016. Taraxacum majoricense (Asteraceae), a new species from the Balearic Islands, Spain. Annales Botanici Fennici 53: 82–90. https://doi.org/10.5735/085.053.0216 |
| 35. |
Generalitat de Catalunya 2008. Decret 172/2008, de 26 d’agost, de creació del Catàleg de flora amenaçada de Catalunya. Diari Oficial de la Generalitat de Catalunya 5204: 65881–65895. |
| 36. |
Generalitat Valenciana 2011. Ordre 2/2011, de 24 de gener, de la Conselleria de Medi Ambient, Aigua, Urbanisme i Habitatge, per la qual es declaren sis noves microreserves de flora a la província de València i es modifiquen les ordres de declaració de microreserves de flora de 4 de maig de 1999, 6 de novembre de 2000, 22 d’octubre de 2002 i 24 d’octubre de 2003. Diari Oficial de la Comunitat Valenciana 6450: 4794–4811. |
| 37. |
Generalitat Valenciana 2016. Espais naturals protegits – Xàtiva. Generalitat Valenciana, València. Retrieved June 12, 2016, from http://www.argos.gva.es/bdmun/pls/argos_mun/DMEDB_MUNDATOSESPNATURALES.DibujaPagina?aNMunId=46145&aVLengua=V |
| 38. |
GOB [Grup Balear d’Ornitologia i defensa de la Naturalesa] 1990. L’Arxipèlag de Cabrera, un parc nacional en litigi. Ed. Moll, Palma de Mallorca. |
| 39. |
Gobierno de España 2010. Resolución 420/38087/2010, de 29 de abril, de la Secretaría General Técnica, por la que se publica el Convenio de colaboración, entre el Ministerio de Defensa y la Consejería de Medio Ambiente de la Comunidad Autónoma de las Illes Balears, para la conservación de la flora vascular en terrenos de Puig Major (escuadrón de vigilancia aérea nº 7 de Soller-Mallorca). Boletín Oficial del Estado 2010(116): 42081–42086. |
| 40. |
Gobierno de España 2015. Orden AAA/1771/2015, de 31 de agosto, por la que se modifica el anexo del Real Decreto 139/2011, de 4 de febrero, para el desarrollo del Listado de Especies Silvestres en Régimen de Protección Especial y del Catálogo Español de Especies Amenazadas. Boletín Oficial del Estado 2015(211): 77925–77929. |
| 41. |
Gorsira, B., Belfit, S. C. & Cantrell, M. A. 1996. Alleviating conflicts between army training and endangered species at Fort Bragg. Federal Facilities Environmental Journal 7: 59–67. https://doi.org/10.1002/ffej.3330070307 |
| 42. |
Grichting, A. 2014. Cyprus: Greening in the dead zone. In: Tidball, K. G. & Krasny, M. E. (Eds.), Greening in the red zone – Disaster, resilience and community greening. Springer, Dordrecht: 429–443. https://doi.org/10.1007/978-90-481-9947-1_33 |
| 43. |
Griffin, M., Boyko, W., Boyko, B. et al. 2001. Integrated cultural resources management plan (ICRMP) for Ft. Bragg, Camp Mackall, and Simmons Army Airfield. Final Draft. Griffin Social Technologies, Chesapeake. |
| 44. |
Grüger, E., Thulin, B., Müller, J., Schneider, J., Alefs, J. & Welter-Schultes, F. W. 2002. Environmental changes in and around Lake Avernus in Greek and Roman times: A study of the plant and animal remains preserved in the lake’s sediments. In: Jashemski, W. F. & Meyer, F. G. (Eds.), The natural history of Pompeii. Cambridge University Press, Cambridge: 240–273. |
| 45. |
Gücel, S., Charalambidou, I., Göçmen, B., Karataş, A., Özden, Ö., Soyumert, A. & Fuller, W. 2008. Monitoring biodiversity of the buffer zone in Cyprus. Poster presented at the “Monitoring biodiversity in Europe. Volunteers, efficiency and cost” congress (Leipzig, Germany, 28–30 January 2008). |
| 46. |
Guitián, J., Sánchez, J. M. & Guitián, P. 1993. Biología y conservación de Petrocoptis grandiflora en el Noroeste Ibérico. Botanica Complutensis 18: 123–128. |
| 47. |
Hanna, W. A. 1978. Indonesian Banda: Colonialism and its aftermath in the Nutmeg Islands. Institute for the Study of Human Issues, Philadelphia. |
| 48. |
Hanson, T., Brooks, T. M. & Da Fonseca, G. A. B. et al. 2009. Warfare in biodiversity hotspots. Conservation Biology 23: 578–587. https://doi.org/10.1111/j.1523-1739.2009.01166.x |
| 49. |
Hassan, R., Scholes, R. & Ash, N. (Eds.) 2005. Ecosystems and human well-being: current state and trends 1. Island Press, Washington. |
| 50. |
Hayes, P. 2010. Sustainable security in the Korean Peninsula: envisioning a northeast Asian biodiversity corridor. Korean Journal of International Studies 8: 197–230. |
| 51. |
Hernàndez, F. X. 2003. Història militar de Catalunya 3. Rafael Dalmau, Barcelona. |
| 52. |
Heywood, V. H. 2013. Award of OPTIMA gold medal to professor Loutfy Boulos Tawadros. Laudatio by professor Vernon Heywood. Organization for the Phyto-Taxonomic Investigation of the Mediterranean Area (OPTIMA), Palermo. Retrieved July 4, 2016, from http://www.optima-bot.org/awards/boulos.htm |
| 53. |
Housset, P. & Lemire, J. 2009. Bilan des stratégies minimales régionales de conservation – Région Haute-Normandie. Le Jouet du Vent 21: 6. |
| 54. |
Howard, R. A. 2000. The role of botanists during World War II in the Pacific Theatre. In: MacLeod, R. (Ed.), Science and the Pacific War – Science and survival in the Pacific, 1939–1945. Kluwer Academic Publishers, Dordrecht: 83–118. |
| 55. |
Hu, C.-M. & Watson, M. F. 2015. Plant exploration in China. In: Hong, D.-Y. & Blackmore, S. (Eds.), Plants of China – A companion to the Flora of China. Science Press, Beijing: 212–236. |
| 56. |
IUCN [International Union for Conservation of Nature] 2012. Resolutions and Recommendations. World Conservation Congress, Jeju, Republic of Korea, 6–15 September 2012. IUCN, Gland. |
| 57. |
IUCN [International Union for Conservation of Nature] 2014. A statement on transboundary conservation for biodiversity and peace. IUCN, Gland. Retrieved March 2, 2016, from http://cmsdata.iucn.org/downloads/biodiversity_and_peace_statement_final_approved_at_cbd_event.pdf |
| 58. |
Jarraud, N. 2008. Hawks, doves and wild-sheep. Development and Transition 9(April): 9–11. |
| 59. |
Junta de Castilla y León 2007. Decreto 63/2007, de 14 de junio, por el que se crean el Catálogo de Flora Protegida de Castilla y León y la figura de protección denominada Microrreserva de Flora. Boletín Oficial de Castilla y León 2007(119): 13197–13204. |
| 60. |
Kanyamibwa, S. 1998. Impact of war on conservation: Rwandan environment and wildlife in agony. Biodiversity and Conservation 7: 1399–1406. https://doi.org/10.1023/A:1008880113990 |
| 61. |
Kim, J.-O., Steiner, F. & Mueller, E. 2011. Cranes, crops and conservation: Understanding human perceptions of biodiversity conservation in South Korea’s Civilian Control Zone. Environmental Management 47: 1–10. https://doi.org/10.1007/s00267-010-9568-1 |
| 62. |
Kim, K. C. 1997. Preserving biodiversity in Korea’s Demilitarized Zone. Science 278: 242–243. https://doi.org/10.1126/science.278.5336.242 |
| 63. |
Kim, K.-G. 2001. A study on the feasibility as well as an operational strategy to develop DMZ Transboundary Biosphere Reserve between DPR Korea and Republic of Korea – Final report. UNESCO Jakarta Office, Jakarta. |
| 64. |
Kim, K.-G. 2013. The Demilitarized Zone (DMZ) of Korea – Protection, conservation and restoration of a unique ecosystem. Springer, Berlin & Heidelberg. |
| 65. |
Kim, K.-G. & Cho, D.-G. 2005. Status and ecological resource value of the Republic of Korea’s De-militarized Zone. Landscape and Ecological Engineering 1: 3–15. https://doi.org/10.1007/s11355-005-0006-0 |
| 66. |
Kim, Y.-S. 2006. Conservation of plant diversity in Korea. Landscape and Ecological Engineering 2: 163–170. https://doi.org/10.1007/s11355-006-0004-x |
| 67. |
Lacan, I. & McBride, J. R. 2009. War and trees: The destruction and replanting of the urban and peri-urban forest of Sarajevo, Bosnia and Herzegovina. Urban Forestry & Urban Greening 8: 133–148. https://doi.org/10.1016/j.ufug.2009.04.001 |
| 68. |
Laguna, E. (Coord.) 1998. Flora endémica, rara o amenazada de la Comunidad Valenciana. Conselleria de Medi Ambient (Generalitat Valenciana), Valencia. |
| 69. |
Laguna, E., Deltoro, V. I., Pérez-Botella, J., Pérez-Rovira, P., Serra, L., Olivares, A. & Fabregat, C. 2004. The role of small reserves in plant conservation in a region of high diversity in eastern Spain. Biological Conservation 119: 421–426. https://doi.org/10.1016/j.biocon.2004.01.001 |
| 70. |
Lefort, A., Marcigny, C., Giraud, P. & Guihard, P.-M. 2012. L’oppidum du Mont-Castel (Port-en-Bessin-Huppain, Calvados). Premiers résultats. Revue Archéologique de l’Ouest 29: 107–132. https://doi.org/10.4000/rao.1801 |
| 71. |
Liu, H., Xu, Z., Xu, Y. & Wang, J. 2002. Practice of conserving plant diversity through traditional beliefs: a case study in Xishuangbanna, southwest China. Biodiversity and Conservation 11: 705–713. https://doi.org/10.1023/A:1015532230442 |
| 72. |
Loades, M. 2013. The longbow. Osprey Publishing, Oxford. |
| 73. |
López-Pujol, J., Font, J., Simon, J. & Blanché, C. 2007. Can the preservation of historical relicts permit the conservation of endangered plant species? The case of Silene sennenii (Caryophyllaceae). Conservation Genetics 8: 903–912. https://doi.org/10.1007/s10592-006-9245-3 |
| 74. |
López-Pujol, J., Martinell, M. C., Massó, S., Blanché, C. & Sáez, L. 2013. The ‘paradigm of extremes’: extremely low genetic diversity in anextremely narrow endemic species, Coristospermum huteri (Umbelliferae). Plant Systematics and Evolution 299: 439–446. https://doi.org/10.1007/s00606-012-0732-3 |
| 75. |
López-Pujol, J., Zhang, F.-M. & Ge, S. 2006. Plant biodiversity in China: richly varied, endangered and in need of conservation. Biodiversity and Conservation 15: 3983–4026. https://doi.org/10.1007/s10531-005-3015-2 |
| 76. |
Loskutov, I. G. 1999. Vavilov and his institute. A history of the world col1ection of plant genetic resources in Russia. International Plant Genetic Resources Institute, Rome. |
| 77. |
Martinell, M. C. 2010. Biología de la conservación de especies amenazadas de área de distribución restringida en Cataluña. PhD Thesis, Universitat de Barcelona, Barcelona. |
| 78. |
Martinell, M. C., Dötterl, S., Blanché, C., Rovira, A., Massó, S. & Bosch, M. 2010. Nocturnal pollination of the endemic Silene sennenii (Caryophyllaceae): an endangered mutualism? Plant Ecology 211: 203–218. https://doi.org/10.1007/s11258-010-9785-y |
| 79. |
Maslo, S. 2014. The urban flora of the city of Mostar (Bosnia and Herzegovina). Natura Croatica 23: 101–145. |
| 80. |
Massó, S., López-Pujol, J., López-Alvarado, J., Blanché, C. & Sáez, L. 2016. One species, one genotype: no genotypic variability in the extremely narrow endemic tetraploid Agrostis barceloi (Gramineae). Plant Systematics and Evolution 302: 609–615. https://doi.org/10.1007/s00606-016-1283-9 |
| 81. |
McNeely, J. A. 2003. Conserving forest biodiversity in times of violent conflict. Oryx 37: 142–152. https://doi.org/10.1017/S0030605303000334 |
| 82. |
Médail, F. & Quézel, P. 1999. Biodiversity hotspots in the Mediterranean Basin: setting global conservation priorities. Conservation Biology 13: 1510–1513. https://doi.org/10.1046/j.1523-1739.1999.98467.x |
| 83. |
MEDINA [Mediterranean Institute for Nature and Anthropos] 2016. The Delos Initiative. MEDINA, Athens. Retrieved July 5, 2016, from http://www.med-ina.org/delos/index.htm |
| 84. |
Merrill, E. D. 1943. Destruction of the Berlin Herbarium. Science 98: 490–491. https://doi.org/10.1126/science.98.2553.490 |
| 85. |
Miller, G. (Ed.) 1996. To the spice islands and beyond: travels in eastern Indonesia. Oxford University Press, New York. |
| 86. |
Mira, E. 2009. Conquista y destrucción de las Indias. Muñoz Moya Editor, Sevilla. |
| 87. |
Miranda, B., Acedo, C. & Llamas, F. 2014. Petrocoptis pyrenaica subsp. viscosa. Fichas con recopilación de información sobre las especies incluidas en el Decreto 63/2007. Consejería de Medio Ambiente (Junta de Castilla y León), Valladolid. |
| 88. |
MNHN [Muséum National d’Histoire Naturelle] 2016. FR1100039 – Batterie de Longues. MNHN, Paris. Retrieved March 12, 2016, from https://inpn.mnhn.fr/espace/protege/FR1100039 |
| 89. |
Mok, J.-M. 2012. UNESCO denies designation of DMZ as Biosphere Reserve: End of hasty efforts neglecting recommendations for North-South joint designation. Kyunghyang Shinmun, July 13, 2012. Retrieved July 6, 2016, from http://english.khan.co.kr/khan_art_view.html?artid=201207131046497&code=710100 |
| 90. |
Montesinos, D. & Güemes, J. 2006. Silene diclinis. The IUCN Red List of Threatened Species 2006: e.T61640A12531397. IUCN, Gland. Retrieved June 30, 2016, from http://dx.doi.org/10.2305/IUCN.UK.2006.RLTS.T61640A12531397.en |
| 91. |
Moragues, E., Mayol, J. & Sáez, L. 2008. Flors del Puig Major. Ed. Perifèric, Palma de Mallorca. |
| 92. |
Moreno, J. C. 2011. La diversidad florística vascular española. Memorias de la Real Sociedad Española de Historia Natural 9: 75–107. |
| 93. |
Mortimer, I. 2009. 1415: Henry V’s year of glory. Random House, London. |
| 94. |
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B. & Kent, J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. https://doi.org/10.1038/35002501 |
| 95. |
Omar, S. A. S., Bhat, N. R. & Asem, A. 2009. Critical assessment of the environmental consequences of the invasion of Kuwait, the Gulf War, and the aftermath. In: Kassim, T. A. & Barceló, D. (Eds.), Environmental consequences of war and aftermath. Springer, Berlin & Heidelberg: 141–170. https://doi.org/10.1007/978-3-540-87963-3_5 |
| 96. |
Öztürk, M., Gucel, S., Guvensen, A., Kadis, C. & Kounnamas, C. 2011. Halophyte plant diversity, coastal habitat types and their conservation status in Cyprus. In: Öztürk, M., Böer, B., Barth, H.-J., Breckle, S.-W., Clüsener-Godt, M. & Khan, M. A. (Eds.), Sabkha ecosystems 3: Africa and Southern Europe. Springer, Dordrecht: 99–111. https://doi.org/10.1007/978-90-481-9673-9 |
| 97. |
Pastoreau, M. 2004. Une histoire symbolique du Moyen Âge occidental. Éditions du Seuil, Paris. |
| 98. |
Pellisier, P & Phelipeau, J. 1980. Los franceses de Cabrera. Ed. Aucadena, Palma de Mallorca. |
| 99. |
Perales, H. R. & Aguirre, J. R. 2008. Biodiversidad humanizada. In: Soberón, J., Halffter, G. & Llorente-Bousquets, J. (Coords.), Capital natural de México 1: Conocimiento actual de la biodiversidad. CONABIO, Ciudad de México: 565–603. |
| 100. |
Rackman, O. 1990. Ancient landscapes. In: Murray, O. & Price, S. (Eds.), The Greek city. From Homer to Alexander. Oxford University Press Oxford: 85–111. |
| 101. |
Redžić, S. 2010. Use of wild and semi-wild edible plants in nutrition and survival of people in 1430 days of siege of Sarajevo during the war in Bosnia and Herzegovina (1992–1995). Collegium Antropologicum 34: 551–570. |
| 102. |
Ricklefs, M. C. 1991. A history of modern Indonesia since c. 1300 (2nd ed.). Macmillan, London. |
| 103. |
Rodas, G. 2009. El funicular del Puig Major cumple 75 años. Diario de Mallorca, April 19, 2009. Retrieved June 26, 2016, from http://www.diariodemallorca.es/actual/2009/04/19/funicular-puig-major-cumple-75-anos/455703.html |
| 104. |
Rudloff, F. V. 1791 The natural history of the nutmeg. Edinburgh Magazine 13: 129–131. |
| 105. |
Sadiq, M. & McCain, J. C. 1993. The Gulf War aftermath – An environmental tragedy. Kluwer Academic Publishers, Dordrecht. https://doi.org/10.1007/978-94-011-1685-5 |
| 106. |
Sáez, L. 2010. Plantes endèmiques dels Països Catalans. In: Giralt, J., Comelles, M., Montagud, E. et al. (Eds.), Suplement Fauna i Flora. Història Natural dels Països Catalans. Enciclopèdia Catalana Barcelona: 161–164. |
| 107. |
Sáez, L., Aymerich, P. & Blanché, C. 2010. Llibre vermell de plantes vasculars endèmiques i amenaçades de Catalunya. Argania Editio, Barcelona. |
| 108. |
Sáez, L. & Rosselló, J. A. 2000. A new species of Agrostis (Gramineae) in the A. alpina complex. Botanical Journal of the Linnean Society 133: 359–370. https://doi.org/10.1111/j.1095-8339.2000.tb01551.x |
| 109. |
Sáez, L. & Rosselló, J. A. 2001. Llibre vermell de la flora vascular de les Illes Balears. Conselleria de Medi Ambient (Govern de les Illes Balears), Palma de Mallorca. |
| 110. |
Sáez, L. & Rosselló, J. A. 2003. Agrostis barceloi L. Sáez & Rosselló. In: Bañares, A., Blanca, G., Güemes, J., Moreno, J. C. & Ortiz, S. (Eds.), Atlas y libro rojo de la flora vascular amenazada de España. Táxones prioritarios. Dirección General de Conservación de la Naturaleza, Madrid: 82–83. |
| 111. |
Salafsky, N., Salzer, D., Stattersfield, A. J. et al. 2008. A standard lexicon for biodiversity conservation: unified classifications of threats and actions. Conservation Biology 22: 897–911. https://doi.org/10.1111/j.1523-1739.2008.00937.x |
| 112. |
Sandwith, T., Shine, C., Hamilton, L. & Sheppard, D. 2001. Transboundary protected areas for peace and co-operation. IUCN, Gland & Cambridge. |
| 113. |
SBAA [Sovereign Base Areas Administration] 2015. Frequently Asked Questions on the designation of Special Areas of Conservation within the Sovereign Base Areas of Akrotiri and Dhekelia. The SBA Administration, Episkopi. Retrieved June 29, 2016, from http://www.sbaadministration.org/images/AEEIC/sac/20151217-ECO_SACs_FAQs_AECO.pdf |
| 114. |
Segura, S. & Torres, J. 2009. Historia de las plantas en el mundo antiguo. CSIC-Universidad de Deusto, Bilbao & Madrid. |
| 115. |
Shetler, S. G. 1967. The Komarov Botanical Institute: 250 years of Russian research. Smithsonian Institutional Press, Washington. https://doi.org/10.5962/bhl.title.46347 |
| 116. |
Solé, R. 1998. Les savants de Bonaparte. Éditions du Seuil, Paris. |
| 117. |
Sorrie, B. A., Van Eerden, B. & Russo, M. J. 1997. Noteworthy plants from Fort Bragg and Camp MacKall, North Carolina. Castanea 62: 239–259. |
| 118. |
Soulé, M. I. 1985. What is conservation biology? BioScience 35: 727–734. https://doi.org/10.2307/1310054 |
| 119. |
Steele, A. R. 1982. Flores para el rey. Ediciones del Serbal, Madrid. |
| 120. |
Sugden, A. M. 2016. Ecological legacy of a civil war. Science 351: 572–573. https://doi.org/10.1126/science.351.6273.572-a |
| 121. |
Sung, C. Y. 2015. Simulation of crane habitat fragmentation in the North and South Korean border region after Korean reunification. Landscape and Urban Planning 134: 10–18. https://doi.org/10.1016/j.landurbplan.2014.10.008 |
| 122. |
Trias-Blasi, A., Gücel, S. & Özden, Ö. 2017. Current distribution and conservation status reassessment of the Cyprus Tulip (Tulipa cypria: Liliaceae), new data from northern Cyprus. Plant Biosystems 151: 394–402. https://doi.org/10.1080/11263504.2016.1174177 |
| 123. |
Tsintides, T., Christodoulou, C. S., Delipetrou, P. & Georghiou, K. (Eds.) 2007. The red data book of the flora of Cyprus. Cyprus Forestry Association, Lefkosia. |
| 124. |
UNESCO 1992–2016. World Heritage List. Archeological site of Delphi. UNESCO World Heritage Centre, Paris. Retrieved July 2, 2016, from http://whc.unesco.org/en/list/393 |
| 125. |
USAEC [US Army Environmental Command] 2007. Draft programmatic environmental impact statement for army growth and force structure realignment of the U.S. Army. US Department of the Army, Washington. |
| 126. |
Vasyliuk, O., Shyriaieva, D., Kolomytsev, G. & Spinova, J. 2017. Steppe protected areas on the territory of Ukraine in the context of the armed conflict in the Donbas region and Russian annexation of the Crimean Peninsula. Bulletin of the Eurasian Dry Grassland Group 33: 15–23. https://doi.org/10.21570/EDGG.Bull.33.15-23 |
| 127. |
Wark, N. J. & Verrier, F. J. 2002. Australian Defence Organisation environmental management initiatives—Shoalwater Bay Training Area. Federal Facilities Environmental Journal 13: 53–63. https://doi.org/10.1002/ffej.10019 |
| 128. |
Weiss, E. A. 2002. Spice crops. CABI Publishing, London & New York. https://doi.org/10.1079/9780851996059.0000 |
| 129. |
Wisner, B. 2001. Risk and the neoliberal state: Why post-Mitch lessons didn’t reduce El Salvador’s earthquake losses. Disasters 25(3): 251–268. https://doi.org/10.1111/1467-7717.00176 |
| 130. |
Yonhap 2014. S. Korea considers reducing civilian control area near DMZ. Yonhap News Agency, May 7, 2014. Retrieved June 21, 2016, from http://english.yonhapnews.co.kr/full/2014/05/07/31/1200000000AEN20140507008700315F.html?76ea0780 |
| 131. |
Zentelis, R. & Lindenmayer, D. 2015. Bombing for biodiversity—enhancing conservation values of military training areas. Conservation Letters 8: 299–305. https://doi.org/10.1111/conl.12155 |